2FIC
CAS Number 2083617-82-5
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers2FIC, high purity building block for non-fullerene acceptors
High quality and expert support available online
Specifications | MSDS | Literature and Reviews
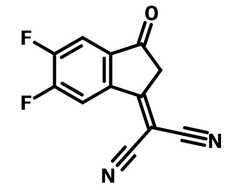
2FIC, namely 2-(5,6-Difluoro-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (CAS number 2083617-82-5), is electron deficient. It is used as a building block to prepare non-fullerene acceptors (NFAs) such as ITIC-2F, IHIC-2F, IEIC-2F, IXIC-2F for highly efficient organic photovoltaic devices.
Fluorination of the end groups can further extend the absorption of the targeted NFAs into near infrared (NIR) due to enhanced intramolecular charge transfer between the core of the NFAs and the end 2FIC groups.
2FIC is strong electron-withdrawing and the electron mobility of the NFAs can be promoted by such intermolecular interactions through forming noncovalent F···S and F···H bonds.
Electron withdrawing building block
For the preparation of non-fullerene acceptors (NFAs)
Fluoride substituent
Expand the absorption of NFAs into near infrared (NIR)
Worldwide shipping
Quick and reliable shipping
Improved electron mobility
Strong intermolecular interactions from halogen-bonding
General Information
| CAS Number | 2083617-82-5 |
| Chemical Formula | C12H4F2N2O |
| Molecular Weight | 230.17 g/mol |
| Full Name | 2-(5,6-Difluoro-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile |
| Synonyms | IC-2F, EG-2F, ID-F, 2-(5,6-Difluoro-2,3-dihydro-3-oxo-1H-inden-1-ylidene)propanedinitrile |
| Classification / Family | Malononitrile, Indanone, Monomer and intermediates, ITIC, None-fullerene acceptors (NFAs), NFA-OSCs, printing electronics |
Chemical Structure
Product Details
| Purity | >98% (by NMR) |
| Melting Point | N/A |
| Appearance | Pale yellow solid |
MSDS Documentation
Literature and Reviews
- Design, Synthesis, and Photovoltaic Characterization of a Small Molecular Acceptor with an Ultra‐Narrow Band Gap, H. Yao et al., Angew. Chem. Int. Ed., 56, 3045 (2017); doi: 10.1002/anie.201610944.
- Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells, W. Zhao et al., J. Am. Chem. Soc., 139(21), 7148-7151 (2017); doi: 10.1021/jacs.7b02677.
- A Twisted Thieno[3,4-b]thiophene-Based Electron Acceptor Featuring a 14-π-Electron Indenoindene Core for High-Performance Organic Photovoltaics, S. Xu et al., Adv. Mater., 1704510 (2017); DOI: 10.1002/adma.201704510.

 2FIC MSDS Sheet
2FIC MSDS Sheet