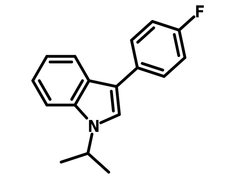
3-(4-Fluorophenyl)-1-isopropylindole
CAS Number 93957-49-4
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated heterocyclic building block
Used as a synthesis intermediate for APIs and dyes in application of drug discovery and photonics
Specifications | MSDS | Literature and Reviews
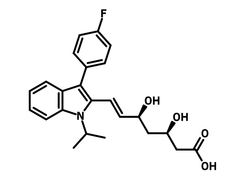
3-(4-Fluorophenyl)-1-isopropylindole (CAS number 93957-49-4) is a derivative of indole, a heterobicyclic compound, with a 4-fluorophenyl (3-position) and an isopropyl substituent (1-position). 3-(4-Fluorophenyl)-1-isopropylindole is the nucleus of Fluvastatin, a drug used to treat hypercholesterolemia for preventing cardiovascular diseases. A series of 3-(4-Fluorophenyl)-1-isopropylindole derivatives are synthesized, presenting a significant decrease of serum total cholesterol, triglycerides, and low-density lipoprotein levels. The syntheses involve a Vilsmeier-Haack reaction for introducing an aldehyde to the 2-position and then a Claisen-Schmidt condensation for the modification of the aldehyde.
3-(4-Fluorophenyl)-1-isopropylindole also shows non-linear optical properties. The electron-withdrawing substituent of indole increases the hyperpolarizability coefficient, thus resulting a better non-linear optical response.
Multiple functional groups
For facile synthesis
Fluorinated indole building block
For drug discovery, biochemistry, and photonics
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 93957-49-4 |
| Chemical Formula | C17H16FN |
| Full Name | 3-(4-Fluorophenyl)-1-isopropyl-1H-indole |
| Molecular Weight | 253.32 g/mol |
| Synonyms | 1-Isopropyl-3-(4-fluorophenyl)indole |
| Classification / Family | Fluorinated building block, heterocyclic building block, APIs, Photonics |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 95 °C – 98 °C |
| Appearance | Off-white to pale beige powder |
MSDS Documentation
 3-(4-Fluorophenyl)-1-isopropylindole MSDS Sheet
3-(4-Fluorophenyl)-1-isopropylindole MSDS Sheet
Literature and Reviews
-
Interaction of indole derivative with human serum albumin: a combined spectroscopic and molecular dynamics study, S. Pawar et al., ChemistrySelect, 3, 12080–12088(2008); DOI: 10.1002/slct.201802466.
-
Optical properties of 3-substituted indoles, J. Kumar et al., RSC Adv., 10, 28213(2020); DOI: 10.1039/d0ra05405d.
- Photoredox cyanomethylation of indoles: catalyst modification and mechanism, O'Brien et al., J. Org. Chem., 83(16), 8926–8935(2018); DOI: 10.1021/acs.joc.8b01146.
