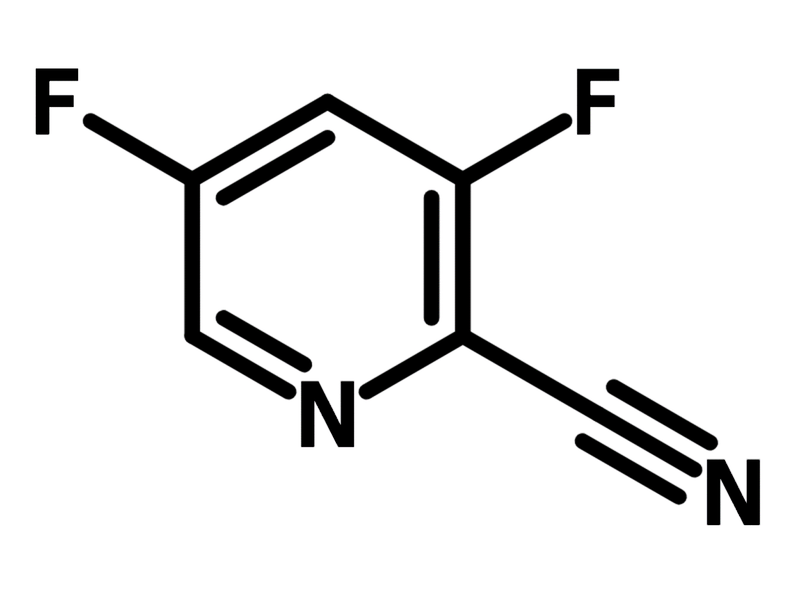
3,5-Difluoropyridine-2-carbonitrile
CAS Number 298709-29-2
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA difluorinated pyridine building block
Serves as a precursor for APIs and TDAF emitters
Specifications | MSDS | Literature and Reviews
3,5-Difluoropyridine-2-carbonitrile (CAS number 298709-29-2), also knowns as 3,5-difluoropicolinonitrile, is a difluorinated cyanopyridine. 3,5-Difluoropyridine-2-carbonitrile is highly electron deficient, thus the fluorine substituents readily undergo nucleophilic aromatic substitution reactions. A thermally activated delayed fluorescence (TADF) emitter, known as 2,4-2(9H-carbazol-9-yl)-1-cyano-pyridine, is prepared using this method, demonstrating maximum current, power and external quantum efficiency values of 27.5 cdA-1, 25.6 lmW-1 and 9.1%, respectively, in OLED application.
3,5-Difluoropyridine-2-carbonitrile finds extensive applications as a molecular scaffold in medicinal chemistry such as the synthesis of thiazole analogues for desferrithiocin.
Multiple functional groups
For facile synthesis
Fluorinated picolinonitrile building block
For drug discovery, TADF dyes and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 298709-29-2 |
| Chemical Formula | C6H2F2N2 |
| Full Name | 3,5-Difluoropyridine-2-carbonitrile |
| Molecular Weight | 140.09 g/mol |
| Synonyms | 3,5-Difluoropicolinonitrile, 2-Cyano-3,5-difluoropyridine |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, APIs, OlEDs, TDAF |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 30 °C – 34 °C |
| Appearance | White crystals |
MSDS Documentation
 3,5-Difluoropyridine-2-carbonitrile MSDS Sheet
3,5-Difluoropyridine-2-carbonitrile MSDS Sheet
Literature and Reviews
- Carbazole/a-carboline hybrid bipolar compounds as electron acceptors in exciplex or non-exciplex mixed cohosts and exciplex-TADF emitters for high-efficiency OLEDs, Q. Wu et al., J. Mater. Chem. C, 6, 8784(2018); DOI: 10.1039/c8tc02353k.
- Structure−activity relationship studies of tolfenpyrad reveal subnanomolar inhibitors of Haemonchus contortus development, T. Le et al., J. Med. Chem., 62(2), 1036–1053(2019); DOI: 10.1021/acs.jmedchem.8b01789.
- Desferrithiocin: a search for clinically effective iron chelators, R. Bergeron et al., J. Med. Chem. 57, 9259–9291(2014); DOI: 10.1021/jm500828f.
