Highly bioactive 10H-phenothiazine derivative
Widely used in DSSCs and energy devices, also for the preparation of hole selective self-assembled monolayers
Specifications | MSDS | Literature and Reviews
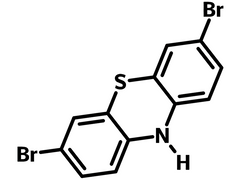
3,7-Dibromo-10H-phenothiazine (37DBrPTz), also known as thiodiphenylamine, is a symmetrically brominated derivate of 10H-phenothiazine. Phenothiazine related compounds are highly bioactive, and its derivatives chlorpromazine and promethazine are well known in the fields of psychiatry and allergy treatment. With a unique non-planar butterfly conformation, the non-conjugated 10H-phenothiazine structure can sufficiently suppress intra-molecular aggregation hence subdue the formation of excimers.
10H-phenothiazine derivatives are also widely used in dye-sensitized solar cells (DSSCs) and energy devices. The electron-rich nitrogen and sulfur atoms render 10H-phenothiazine a stronger donor character than other amines, i.e. triphenylamine, tetrahydroquinoline and carbazole. PTZAN||Zn battery based on 4,4′-(10H-phenothiazine-3,7-diyl) bis (N,N-diphenylaniline) (PTZAN), showed excellent stability (2000 cycles), high voltage (1.3 V), high capacity (145 mAh g−1), and energy density of 187.2 Wh kg−1.
Most recently, 3,7-Dibromo-10H-phenothiazine has been engaged in the synthesis of self-assembled monolayer (SAM) materials such as Br-2EPT that was serving as the hole selective contact between anode and perovskite active layer. Perovskite solar cells using Br-2EPT as the interface achieved a power conversion efficiency (PCE) of up to 22.44% (certified 21.81%) with an average fill factor close to 81%, one of the top efficiencies reported to date for p-i-n PSCs.
General Information
| CAS Number | 21667-32-3 |
|---|---|
| Chemical Formula | C12H7Br2NS |
| Full Name | 3,7-Dibromo-10H-phenothiazine |
| Molecular Weight | 357.07 g/mol |
| Synonyms | 37DBrPTz, Thiodiphenylamine, Dibenzothiazine, Dibenzoparathiazine |
| Classification or Family | 10H-phenothiazine, Heterocyclic building blocks, SAMs |
Chemical Structure
Product Details
| Purity | > 98% |
|---|---|
| Melting Point | - |
| Appearance | Pale green to beige powder |
MSDS Documentation
 3,7-Dibromo-10H-phenothiazine MSDS Sheet
3,7-Dibromo-10H-phenothiazine MSDS Sheet
We stock a wide range of COF ligands available to purchase online. Please contact us if you cannot find what you are looking for.
Literature and Reviews
-
Synthesis and characterization of thermally cross-linkable hole injection polymer based on poly(10-alkylphenothiazine) for polymer light-emitting diode, M. Jung et al., Synth. Met., 159, 1928-1933 (2009); DOI: 10.1016/j.synthmet.2009.05.034.
-
Molecular Tailoring of p–type Organics for Zinc Batteries with High Energy Density, X. Qiu et al., Angew. Chem. Int. Ed., 62 (30), e202304036 (2023); DOI: 10.1002/anie.202304036.
-
Dual-channel D-(π-A)2 phenoxazine/phenothiazine dyes with an auxiliary N-alkoxy benzoic acid anchor for fabrication of dye-sensitized solar cells, Sol. Energy, 225, 173-183 (2021); DOI: 10.1016/j.solener.2021.07.030.
-
Novel Phenothiazine-Based Self-Assembled Monolayer as a Hole Selective Contact for Highly Efficient and Stable p-i-n Perovskite Solar Cells, A. Ullah et al., Adv. Energy Mater., 2103175 (2021);DOI: DOI: 10.1002/aenm.202103175.
-
Simple and robust phenoxazine phosphonic acid molecules as self-assembled hole selective contacts for high-performance inverted perovskite solar cells, Z. Li et al., Nanoscale, 15, 1676-1686 (2023); DOI: 10.1039/D2NR05677A.
