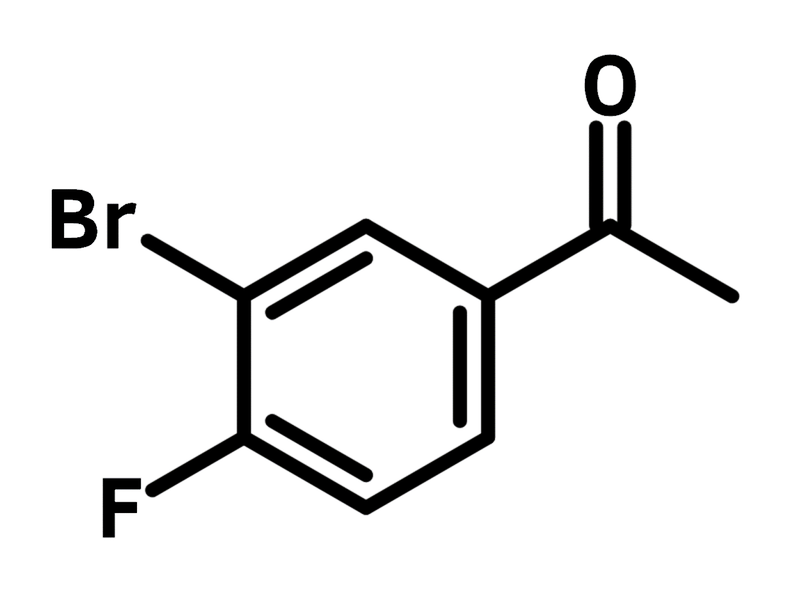
3′-Bromo-4′-fluoroacetophenone
CAS Number 1007-15-4
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksA dihalogenated acetophenone building block
As a synthesis intermediate for APIs, chalcone derivatives and carbazole derivatives
Specifications | MSDS | Literature and Reviews
3′-Bromo-4′-fluoroacetophenone (CAS number 1007-15-4) is an acetophenone derivative featuring meta-bromide and para-fluoride function groups. Para/meta-substituted acetophenones have shown antimicrobial activity with inhibitory concentration as low as 246 µM. 3′-Bromo-4′-fluoroacetophenone is applied as a precursor for the synthesis of chalcone, an α,β-unsaturated ketone that is utilised in drug discovery, liquid crystals and optical materials.
3′-Bromo-4′-fluoroacetophenone is also a precursor for an acetyl-functionalised carbazole through a tandem Suzuki-coupling/SNAr approach.
Multiple functional groups
For facile synthesis
Fluorinated acetophenone building block
For drug discovery, chalcone derivatives, and carbazole derivatives
Low Cost
Competitively priced, high quality product
High purity
>98% High purity
General Information
| CAS Number | 1007-15-4 |
| Chemical Formula | C8H6BrFO |
| Full Name | 1-(3-Bromo-4-fluorophenyl)ethanone |
| Molecular Weight | 217.04 g/mol |
| Synonyms | 1-(3-Bromo-4-fluorophenyl)ethan-1-one, 1-Acetyl-3-bromo-4-fluorobenzene |
| Classification / Family | Fluorinated building blocks, Brominated building blocks, Acetophenone building blocks, APIs, Chalcone precursors, Carbazole precursors |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 52 °C – 57 °C |
| Appearance | Yellow/light brown powder/crystals |
MSDS Documentation
 3′-Bromo-4′-fluoroacetophenone MSDS Sheet
3′-Bromo-4′-fluoroacetophenone MSDS Sheet
Literature and Reviews
- Large enhancement of nonlinear optical absorption and refraction in planar chalcone structure with terminal substitution, J. Chen et al., Opt. Mater., 146, 114519 (2023); DOI: 10.1016/j.optmat.2023.114519.
- A tandem cross-Coupling/SNAr approach to functionalized carbazoles, D. St. Jean et al, Org. Lett., 9 (23), 4893–4896 (2007); DOI: 10.1021/ol702274y.
- Acetophenones with selective antimycobacterial activity, L. Rajabi et al., Lett. Appl. Microbiol., 40, 212–217 (2005); DOI: 10.1111/j.1472-765X.2005.01657.x.
