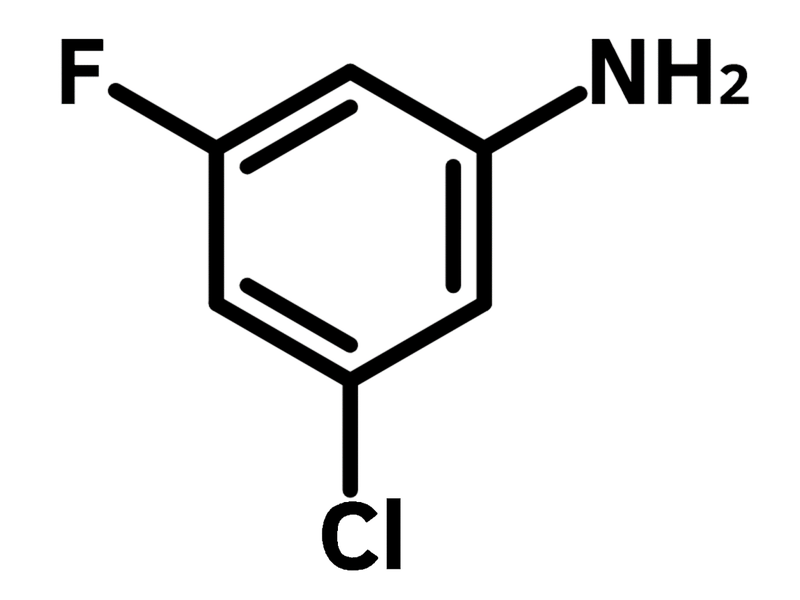
3-Chloro-5-fluoroaniline
CAS Number 4863-91-6
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA fluorinated aniline building block
For the synthesis of APIs for inflammatory and antiviral treatments
Specifications | MSDS | Literature and Reviews
3-Chloro-5-fluoroaniline (CAS number 4863-91-6) is an aniline derivative bearing chlorine and fluorine substituents at the 3- and 5-positions. 3-Chloro-5-fluoroaniline is extensively used to synthesise active pharmaceutical ingredients (APIs), particularly antiviral compounds targeting the influenza A H1N1 virus. 3-Chloro-5-fluoroaniline can be readily incorporated into molecular scaffolds through reductive amination and nucleophilic substitution. 3-Chloro-5-fluoroaniline is also involved in the synthesis of glucocorticoid receptor agonist for inflammation treatments. The synthesis of the tetrahydroquinoline-based glucocorticoid receptor agonist commences with 3-chloro-5-fluoroaniline reacting with acetone and iodine under Skraup reaction.
The presence of chlorine and fluorine substituents in 3-chloro-5-fluoroaniline enables further functionalisation, such as palladium-catalysed carbon-carbon coupling and nucleophilic aromatic substitution.
Multiple functional groups
For facile synthesis
Fluorinated aniline building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 4863-91-6 |
| Chemical Formula | C6H5ClFN |
| Full Name | 3-Chloro-5-fluoroaniline |
| Molecular Weight | 145.56 g/mol |
| Synonyms | 3-Chloro-5-fluorobenzenamine |
| Classification / Family | Fluorinated building blocks, Aniline building blocks, APIs |
Chemical Structure
Product Details
| Purity | 97% |
| Boiling Point | Tb = 226 °C at 760 mmHg |
| Relative Density | 1.45 g/mL |
| Appearance | Clear liquid |
MSDS Documentation
 3-Chloro-5-fluoroaniline MSDS Sheet
3-Chloro-5-fluoroaniline MSDS Sheet
Literature and Reviews
- Aniline-based inhibitors of influenza H1N1 virus acting on hemagglutinin-mediated fusion, R. Leiva et al., J. Med. Chem., 61, 98–118(2018); DOI: 10.1021/acs.jmedchem.7b00908.
- Discovery of orally available tetrahydroquinoline-based glucocorticoid receptor agonists, Bioorg. Med. Chem. Lett., 21, 1697–1700(2011); DOI: 10.1016/j.bmcl.2011.01.093.
- Species-selective pyrimidineamine inhibitors of Trypanosoma brucei S-adenosylmethionine decarboxylase, O. Volkov et al., J. Med. Chem., 61(3); DOI: 10.1021/acs.jmedchem.7b01654.
