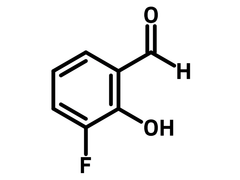
3-Fluoro-2-hydroxybenzaldehyde
CAS Number 394-50-3
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA fluorinated hydroxybenzaldehyde building block
For synthesizing salen/salalen ligands, bicyclic heterocycles and macromolecules in application of APIs, semiconductors and gas storage
Specifications | MSDS | Literature and Reviews
3-Fluoro-2-hydroxybenzaldehyde (CAS number 394-50-3), also known as 3-Fluorosalicylaldehyde, is a benzaldehyde derivative with a fluorine at 3-position and a hydroxy at 2-position. 3-Fluoro-2-hydroxybenzaldehyde is commonly used to synthesize salen (salicylaldehyde and ethylenediamine) ligands as tetradentate C2-symmertic ligands. Cobalt-salen complexes based on 3-fluoro-2-hydroxybenzaldehyde show reversible oxygen chemisorption as well as anticancer activity with IC50 (half maximal inhibitory concentration) of 50 µM.
By having three chemically reactive sites, 3-fluoro-2-hydroxybenzaldehyde is used to synthesize fluorinated bicyclic heterocycles such as isoflavanone (chromanone) through gold(I) catalysed annulation reaction. Semiconducting acenes can also be prepared from 3-fluoro-2-hydroxybenzaldehyde.
Multiple functional groups
For facile synthesis
Fluorinated salicylaldehyde building block
For drug discovery, organometallic ligands and semiconducting materials
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 394-50-3 |
| Chemical Formula | C7H5FO2 |
| Full Name | 3-Fluoro-2-hydroxybenzaldehyde |
| Molecular Weight | 140.11 g/mol |
| Synonyms | 3-Fluorosalicylaldehyde, 2-Hydroxy-3-fluorobenzaldehyde |
| Classification / Family | Fluorinated building block, Salicylaldehyde building block, Schiff bases, APIs, Semiconductors, Gas storage |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 68 – 70 °C |
| Appearance | White to off-white powder |
MSDS Documentation
 3-Fluoro-2-hydroxybenzaldehyde MSDS Sheet
3-Fluoro-2-hydroxybenzaldehyde MSDS Sheet
Literature and Reviews
-
Regioselective fluorination of acenes: tailoring of molecular electronic levels and solid-state properties, D. Bischof et al., Chem. Eur. J., 28, e202103653(2022); DOI: 10.1002/chem.202103653.
-
Investigation of fluorinated and bifunctionalized 3-phenylchroman-4-one (isoflavanone) aromatase inhibitors, E. Amato et al., Bioorg. Med. Chem., 22(1), 126–134(2014); DOI: 10.1016/j.bmc.2013.11.045.
-
Physical properties, ligand substitution reactions, and biological activity of Co(III)-Schiff base complexes, A. King et al., Dalton Trans., 48, 5987–6002(2019); DOI: 10.1039/C8DT04606A.
