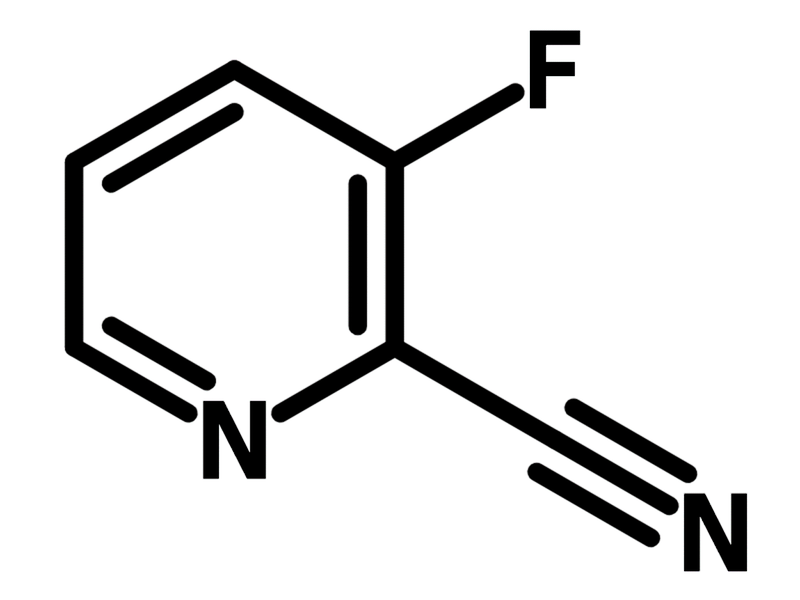
3-Fluoro-2-pyridinecarbonitrile
CAS Number 97509-75-6
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated pyridinecarbonitrile building block
Used as a synthesis intermediate for APIs and phosphorescence dyes
Specifications | MSDS | Literature and Reviews
3-Fluoro-2-pyridinecarbonitrile (CAS number 97509-75-6) is a pyridine derivative having a fluorine and a nitrile group at 3- and 2-positions. 3-Fluoro-2-pyridinecarbonitrile can undergo different reactions, such as nucleophilic aromatic substitutions on the fluorine substituted carbon. It allows 3-fluoro-2-pyridinecarbonitrile to attach to chromophores like carbazoles, for synthesising aggregation-induced emission molecules. Such method is also applied to pharmaceutical compounds, by attaching 3-fluoro-2-pyridinecarbonitrile to negative allosteric modulator small molecules leading to an improved binding affinity to glutamate receptors for the treatment of central nervous system diseases.
3-Fluoro-2-pyridinecarbonitrile is also used to prepare tetrazine through bioorthogonal chemistry with hydrazine. The as-synthesised tetrazines facilitate additional reactions, including tetrazine ligation and [4+1] cycloaddition.
Multiple functional groups
For facile synthesis
Fluorinated picolinonitrile building block
For AIE materials, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 97509-75-6 |
| Chemical Formula | C6H3FN2 |
| Full Name | 3-Fluoro-2-pyridinecarbonitrile |
| Molecular Weight | 122.10 g/mol |
| Synonyms | 2-Cyano-3-fluoropyridine, 3-fluoropicolinonitrile, 3-Fluoro-pyridine-2-carbonitrile |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, APIs, Aggregation-induced emission materials, Phosphorescence |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 27 °C – 32 °C |
| Appearance | White crystals |
MSDS Documentation
 3-Fluoro-2-pyridinecarbonitrile MSDS Sheet
3-Fluoro-2-pyridinecarbonitrile MSDS Sheet
Literature and Reviews
- Enabling in vivo photocatalytic activation of rapid bioorthogonal chemistry by repurposing silicon-rhodamine fluorophores as cytocompatible far-red photocatalysts, C. Wang et al., J. Am. Chem. Soc., 143(28), 10793–10803(2021); DOI: 10.1021/jacs.1c05547.
- Sonogashira cross-coupling reaction of bromocyanofluoropyridine nuclei: access to 5- and 6-alkynylfluoropyridinamidoximes scaffolds, F. Razafindrainibe et al., Eur. J. Org. Chem., 2021, 4393(2021); DOI: 10.1002/ejoc.202100563.
- A readily obtained alternative of 1H-benzo[f]indole towards room temperature ultralong organic phosphorescence, X. Fu et al., Chem. Mater., 35(1), 347–357(2023); DOI: 10.1021/acs.chemmater.2c03484.
