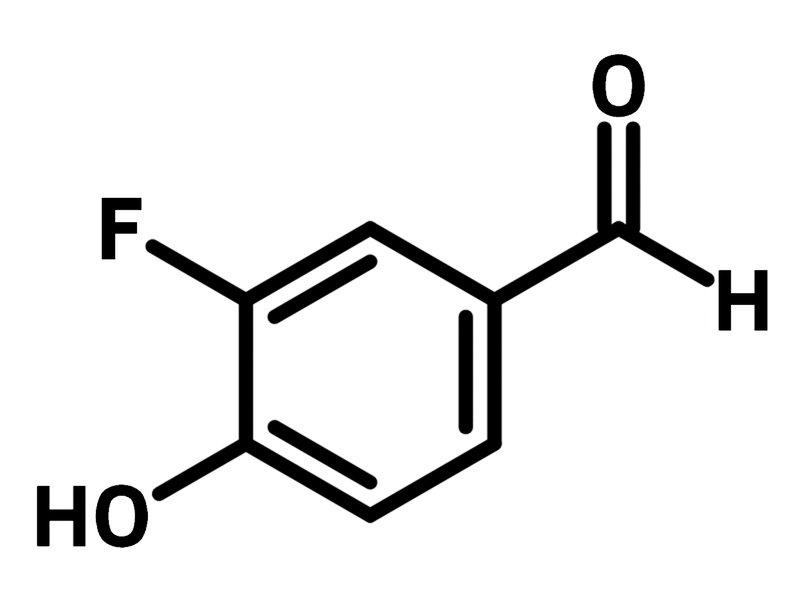
3-Fluoro-4-hydroxybenzaldehyde
CAS Number 405-05-0
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA fluorinated benzaldehyde building block
Used as a synthesis intermediate for chalcones and curcumins in application of pharmaceutical active ingredients
Specifications | MSDS | Literature and Reviews
3-Fluoro-4-hydroxybenzaldehyde (CAS number 405-05-0) is a fluorinated benzaldehyde bearing a fluorine and a hydroxy at 3- and 4-positions. 3-Fluoro-4-hydroxybenzaldehyde is used to synthesise analogues of curcuminoid with ketones through aldol condensation. The as synthesised curcuminoid derivatives show half maximal inhibitory concentration of 0.75 μM against human ovarian cancer cell line A2780. Hydrazone derivatives can be obtained from 3-fluoro-4-hydroxybenzaldehyde with a phenylhydrazine derivatives via aldehyde-amine condensation, showing potent inhibitory of macrophage migration in anti-inflammatory activity.
3-Fluoro-4-hydroxybenzaldehyde is also exploited to synthesise caffeic acid phenylethyl amide derivatives through Wittig reaction for cytoprotective activity against peroxides.
Multiple functional groups
For facile synthesis
Fluorinated benzaldehyde building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 405-05-0 |
| Chemical Formula | C7H5FO2 |
| Full Name | 3-Fluoro-4-hydroxybenzaldehyde |
| Molecular Weight | 140.11 g/mol |
| Synonyms | 2-Fluoro-4-formylphenol |
| Classification / Family | Fluorinated building blocks, Benzaldehyde building blocks, APIs |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 121 °C – 124 °C |
| Appearance | Beige powder |
MSDS Documentation
 3-Fluoro-4-hydroxybenzaldehyde MSDS Sheet
3-Fluoro-4-hydroxybenzaldehyde MSDS Sheet
Literature and Reviews
- Synthesis of a series of caffeic acid phenethyl amide (CAPA) fluorinated derivatives: Comparison of cytoprotective effects to caffeic acid phenethyl ester (CAPE), J. Yang et al., Bioorg. Med. Chem., 18, 5032–5038(2010); DOI: 10.1016/j.bmc.2010.05.080.
- Novel arylazoarylmethane as potential inhibitor of macrophage migration inhibitory factor, M. He et al., Arch. Pharm. Chem. Life Sci., 346, 1–4(2013); DOI: 10.1002/ardp.201300243.
- Enzymatic Baeyer-Villiger Oxidation of Benzaldehydes, M. Moonen et al., Adv. Synth. Catal., 347, 1027–1034(2005); DOI: 10.1002/adsc.200404307.
