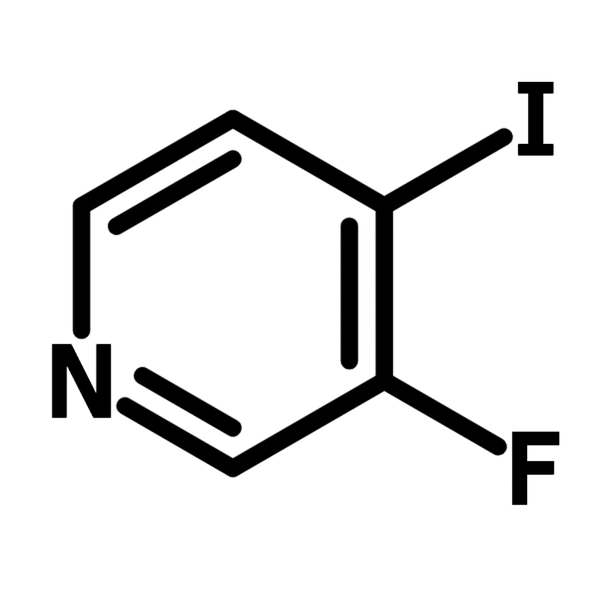
3-Fluoro-4-iodopyridine
CAS Number 22282-75-3
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated pyridine building block
Used as a synthesis intermediate for APIs and synthetic ligands
Specifications | MSDS | Literature and Reviews
3-Fluoro-4-iodopyridine (CAS number 22282-75-3) is a pyridine derivative with a fluoride and an iodide at 3- and 4-positions, respectively. This dihalogenated heterocyclic building block is commonly used as a molecular scaffold for active pharmaceutical ingredients (APIs) and a substrate for synthetic chemistry. The synthesis of an antibiotic Eudistomin T involves the Suzuki coupling reaction of 3-fluoro-4-iodopyridine with (2-pivaloylaminophenyl)boronic acid, followed by lithiation to yield the trisubstituted pyridine. A cyclization reaction is then carried out to complete the formation of Eudistomin T.
3-Fluoro-4-iodopyridine is one of the key building blocks for the synthesis of β-carboline. β-Carboline finds extensive applications in anti-cancer treatments, neuroenhancement and neuroprotection.
Multiple functional groups
For facile synthesis
Fluorinated pyridine building block
For drug discovery, medicinal chemistry, and organic synthesis
Low Cost
Competitively priced, high quality product
High purity
>97% High purity
General Information
| CAS Number | 22282-75-3 |
| Chemical Formula | C5H3FIN |
| Full Name | 3-Fluoro-4-iodopyridine |
| Molecular Weight | 222.99 g/mol |
| Synonyms | 4-Iodo-3-fluoropyridine |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Pyridine building blocks, APIs, Organic synthesis |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 85 °C – 89 °C |
| Appearance | Off-White Powder/Fibres |
MSDS Documentation
 3-Fluoro-4-iodopyridine MSDS Sheet
3-Fluoro-4-iodopyridine MSDS Sheet
Literature and Reviews
- Carbolines, part VIII. An original synthesis of the antibiotic Eudistomin T, P. Rocca et al., Synth. Commun., 25 (32), 3373–3379 (1995); DOI: 10.1080/00397919508013858.
- Continuous-flow divergent lithiation of 2,3-dihalopyridines: deprotolithiation versus halogen dance, T. Brégent et al., Chem. Eur. J., 28, e202202286 (2022); DOI: 10.1002/chem.202202286.
- Engineering hydrogen bonding to align molecular dipoles in organic solids for efficient second harmonic generation, R. Zhao et al., Chem. Sci., 13, 12144 (2022); DOI: 10.1039/d2sc03994j.
