3-(Trifluoromethyl)-2-pyridone
CAS Number 22245-83-6
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated heterocyclic building block
Used as a synthesis intermediate for ligands and bioactive ingredients in application of drug discovery and catalytic reactions
Specifications | MSDS | Literature and Reviews
3-(Trifluoromethyl)-2-pyridone (CAS number 22245-83-6), also known as 2-hydroxy-3-(trifluoromethyl)pyridine, is a trifluoromethyl substituted 2-hydroxypyridine. In catalytic reactions, the amide group of 2-pyridone binds to transition metals forming stable 5-/6-membred ring intermediates. Therefore, 3-(trifluoromethyl)-2-pyridone is extensively used as ligands in catalytic reaction such as directed C-H activation on meta-position. The trifluoromethyl substituent of 3-(trifluoromethyl)-2-pyridone tunes its metal-binding affinity and limits the reaction site for improving regioselectivity.
Many derivatives of 2-pyridone are bioactive such as Doravirine a non-nucleoside reverse transcriptase inhibitor for treatment of AIDS. An imidazoline derivative containing 2-pyridone is evaluated as neuropeptide-Y receptor Y5 antagonists and it displays significant inhibition of food intake for treatment of diet-induced obesity.
Multiple functional groups
For facile synthesis
Fluorinated pyridone building block
For drug discovery, ligands, and organic synthesis
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 22245-83-6 |
| Chemical Formula | C6H4F3NO |
| Full Name | 2-Hydroxy-3-(trifluoromethyl)pyridine |
| Molecular Weight | 163.10 g/mol |
| Synonyms | 3-(Trifluoromethyl)pyridin-2-ol |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Ligands, Catalytic reactions, APIs |
Chemical Structure
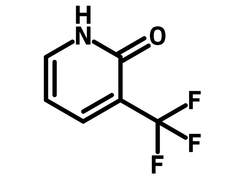
Product Details
| Purity | 97% |
| Melting Point | Tm = 150 °C – 155 °C |
| Appearance | Off-white to pale purple powder |
MSDS Documentation
 3-(Trifluoromethyl)-2-pyridone MSDS Sheet
3-(Trifluoromethyl)-2-pyridone MSDS Sheet
Literature and Reviews
-
De novo generation of a bright blue fluorophore from 2-oxoglutarate in biological samples, Y. Kim et al., Chem. Sci., 13, 365(2022); DOI: DOI: 10.1039/d1sc05808h.
- Discovery of pyridone-containing imidazolines as potent and selective inhibitors of neuropeptide Y Y5 receptor, M. Ando et al., Bioorg. Med. Chem., 17, 6106–6122(2009); DOI: 10.1016/j.bmc.2009.05.069.
-
Ligand-promoted meta-C-H functionalization of benzylamines, P. Wang et al., Angew. Chem. Int. Ed. Engl., 56(18), 5125–5129(2017); DOI: 10.1002/anie.201701803.
