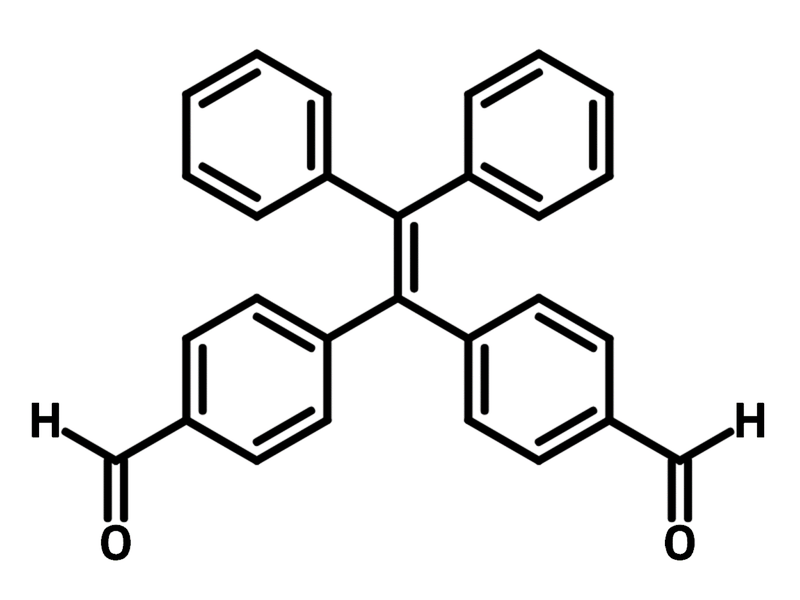
4,4'-(2,2-Diphenylethene-1,1-diyl)dibenzaldehyde
CAS Number 1601465-06-8
Carbaldehyde Monomers, Chemistry Building Blocks, COF Ligands, Materials, Monomers, Porous Organic FrameworksCovalent organic frameworks (COFs) tetraphenylethylene ligand
to synthesise COF for electrocatalysis and AIE materials
Specifications | MSDS | Literature and Reviews
4,4'-(2,2-Diphenylethene-1,1-diyl)dibenzaldehyde (CAS number 1601465-06-8), is a tetraphenylethylene-based ligand with two aldehyde groups. The tetraphenylethylene core consists of four benzene ring connected by a ethene, making 4,4'-(2,2-diphenylethene-1,1-diyl)dibenzaldehyde highly conjugated. Therefore, 4,4'-(2,2-diphenylethene-1,1-diyl)dibenzaldehyde has been employed in combination with benzylamine to synthesise aggregation-induced emission (AIE) molecules through reductive amination.
A one-dimensional COF (1D-COFs) can be prepared from 4,4'-(2,2-diphenylethene-1,1-diyl)dibenzaldehyde and its amine derivatives with imine linkages. The resulting 1D COF displays good crystallinity, high surface areas and excellent chemical stability for electrocatalytic oxygen reduction reaction. It exhibits a high H2O2 selectivity of 85.8 % in the oxygen reduction reaction, with a catalytic turnover frequency (TOF) value of 0.051 s−1 at 0.2 V.
Facile reactions
Aldehyde possesses excellent reactivity
High Purity
>98% Purity
Worldwide shipping
Quick and reliable shipping
MOF and COF ligands
Aldehyde ligand for cross-linked COF networks
General Information
| CAS Number | 1601465-06-8 |
| Chemical Formula | C28H20O2 |
| Full Name | 4,4'-(2,2-Diphenyl-1,1-ethenediyl)dibenzaldehyde |
| Molecular Weight | 388.45 g/mol |
| Synonyms | N/A |
| Classification / Family | COFs, CMPs, Aldehyde ligands, Electrocatalysts, 1D-COFs, AIEs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | N/A |
| Appearance | Off-white powder |
MSDS Documentation
 4,4'-(2,2-Diphenylethene-1,1-diyl)dibenzaldehyde MSDS Sheet
4,4'-(2,2-Diphenylethene-1,1-diyl)dibenzaldehyde MSDS Sheet
Literature and Reviews
- Synthesis of isoniazid-substituted tetraphenylethylene stereoisomers with dramatic differences on aggregate morphologies, optical and mechanochromic properties, Z. Lu et al., J. Photochem. Photobiol. A, 392, 112357 (2020); DOI: 10.1016/j.jphotochem.2020.112357.
- A self-assembling induced emission system constructed by host-guest interaction of AIE-active building blocks, W. Bai et al., Chem. Commun., 51, 1089-1091 (2015); DOI: 10.1039/C4CC06510G.
- One-dimensional covalent organic frameworks for the 2e- oxygen reduction reaction, S. An et al., Angew. Chem. Int. Ed., 62, e202218742 (2023); DOI: 10.1002/anie.202218742.
