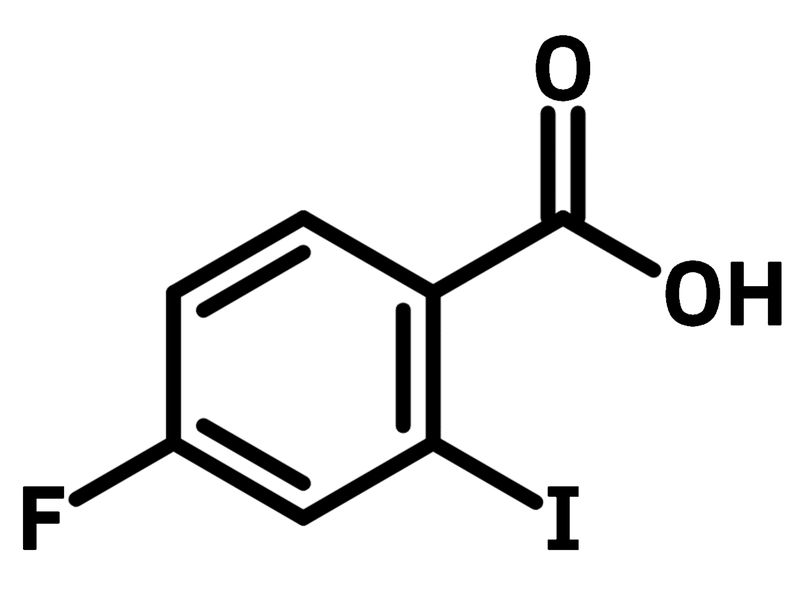
4-Fluoro-2-iodobenzoic acid
CAS Number 56096-89-0
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Non-Heterocyclic Building BlocksA fluorinated benzoic acid building block
Used as a molecular scaffold for organic synthesis
Specifications | MSDS | Literature and Reviews
4-Fluoro-2-iodobenzoic acid (CAS number 56096-89-0) is a dihalogenated benzoic acid with a fluoride sitting opposite and an iodide next to the carboxylic acid around the hexagonal table of a benzene ring. With its three different functional groups, 4-fluoro-2-iodobenzoic acid serves as a versatile building block. As a result, bioactive bicyclic heterocycles such as phthalide and isocoumarin can be synthesised, using a Sonogashira-type reaction. Isocoumarin as the 6-endo-dig product (according to Balwin’s rule) is selectively formed at 100 °C, whereas phthalide, the 5-exo-dig product, is favoured at a lower temperature of 25 °C.
4-Fluoro-2-iodobenzoic acid is also employed in the synthesis of pseudocyclic benziodoxole tosylates as a hypervalent iodine oxidant for organic synthesis. The product is prepared via a ligand transfer reaction between 4-fluoro-2-iodobenzoic acid and PhI(OH)OTs.
Multiple functional groups
For facile synthesis
Fluorinated benzoic acid building block
For drug discovery, medicinal chemistry, and organic synthesis
Low Cost
Competitively priced, high quality product
High purity
>98% High purity
General Information
| CAS Number | 56096-89-0 |
| Chemical Formula | C7H4FIO2 |
| Full Name | 4-Fluoro-2-iodobenzoic acid |
| Molecular Weight | 266.01 g/mol |
| Synonyms | 2-Iodo-4-fluorobenzoic acid |
| Classification / Family | Fluorinated building blocks, Benzoic acid building blocks, Iodide building blocks, Molecular scaffolds, Organic synthesis, Hypervalent iodine oxidants |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 143 °C – 147 °C |
| Appearance | Off-white powder |
MSDS Documentation
 4-Fluoro-2-iodobenzoic acid MSDS Sheet
4-Fluoro-2-iodobenzoic acid MSDS Sheet
Literature and Reviews
- Preparation, structure, and reactivity of pseudocyclic benziodoxole tosylates: new hypervalent iodine oxidants and electrophiles, A. Yoshimura et al., Chem. Eur. J., 23, 691 (2017); DOI: 10.1002/chem.201604475.
- Enantioselective hydroamination of inactivated terminal alkenes, S. Ma et al., Chem., 8, 532–542 (2022); DOI: 10.1016/j.chempr.2021.12.005.
- Chemo- and regioselective benzylic C(sp3)–H oxidation bridging the gap between hetero- and homogeneous copper catalysis, S. Nandi et al., iScience, 25, 104341 (2022); DOI: 10.1016/j.isci.2022.104341.
