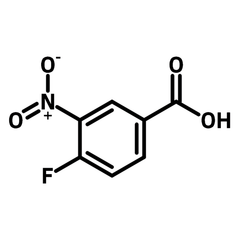
4-Fluoro-3-nitrobenzoic acid
CAS Number 453-71-4
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA fluorinated building block with multiple functional groups
A versatile building block to synthesize benzimidazoles, benzoselenazoles and polymersomes for APIs and fluorescent dyes
Specifications | MSDS | Literature and Reviews
4-Fluoro-3-nitrobenzoic acid (CAS number 453-71-4) is derived from benzoic acid having a nitro and a fluoro substituents at 3- and 4-positions. The functional groups in 4-fluoro-3-nitrobenzoic acid possess different reactivities. The carboxylic acid reacts with alcohols for esters. Fluoride substituent favours nucleophilic aromatic substitution while nitro group can be reduced to an amine as a nucleophile. The ortho-position of fluoride and nitro makes 4-fluoro-3-nitrobenzoic acid an ideal precursor for making benzimidazoles derivatives. Benzimidazoles are active pharmaceutical ingredients applied as antimicrobials, opioids, antipsychotics and antihistamines. Benzoselenazoles (similar to benzimidazole, one nitrogen is replaced by a selenium atom) can be synthesized from 4-fluoro-3-nitrobenzoic acid reacting with isoselenocyanate.
It is feasible to attach various polymers to 4-fluoro-3-nitrobenzoic acid for block-co-polymers, in order to construct polymersomes.
Multiple functional groups
For facile synthesis
Fluorinated benzoic acid building block
For drug discovery, fluorescent dyes, and organic synthesis
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 453-71-4 |
| Chemical Formula | C7H4FNO4 |
| Full Name | 4-Fluoro-3-nitrobenzoic acid |
| Molecular Weight | 185.11 g/mol |
| Synonyms | N/A |
| Classification / Family | Fluorinated building block, APIs, Fluorescent dyes |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 123 – 126 °C |
| Appearance | White to off-white powder |
MSDS Documentation
 4-Fluoro-3-nitrobenzoic acid MSDS Sheet
4-Fluoro-3-nitrobenzoic acid MSDS Sheet
Literature and Reviews
-
Antituberculosis: synthesis and antimycobacterial activity of novel benzimidazole derivatives, Y. Yoon et al., Biomed Res. Int., 2013, 926309(2013); DOI: 10.1155/2013/926309.
-
Discovery of a potent and highly fluorescent sirtuin inhibitor, Y. Yoon et al., Med. Chem. Commun., 6, 1857–1863(2015); DOI: 10.1039/C5MD00307E.
-
Leuko-polymersomes, D. Hammer et al., Faraday Discuss., 139, 129-141(2008); DOI: 10.1039/B717821B.
