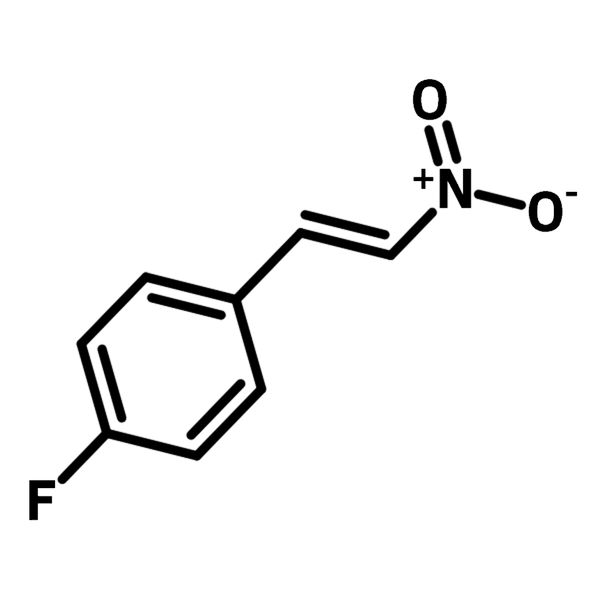
4-Fluoro-β-nitrostyrene
CAS Number 706-08-1
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksA fluorinated nitrostyrene building block
Used as a Michael acceptor in conjugated addition for chromenes/benzopyran for APIs
Specifications | MSDS | Literature and Reviews
4-Fluoro-β-nitrostyrene (CAS number 706-08-1) is a fluorinated styrene with a nitro group substituted on the β-carbon. 4-Fluoro-β-nitrostyrene is a typical Michael acceptor with the electron withdrawing nitro group, which is readily for conjugated addition. When the conjugated addition is catalysed by a chiral ligand, such as l-proline, syn-addition is favoured, and the chirality of the Michael adducts can be selectively controlled. Hence, 4-fluoro-β-nitrostyrene has been exploited for chiral γ-nitroaldehydes, a precursor for pharmaceutically active γ-aminobutyric acid (GABA). 4-Fluoro-β-nitrostyrene is also used to synthesise benzopyran(chromenes) derivatives as pharmaceutical active ingredients(APIs).
In antibacterial activity study, 4-fluoro-β-nitrostyrene binds to PTP1B enzymes via hydrogen bonding interaction. It is followed by a Michael addition of thiol groups leading to the inhibition of protein tyrosine phosphatases in the microorganisms.
Multiple functional groups
For facile synthesis
Fluorinated nitrostyrene building block
For organic synthesis, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 706-08-1 |
| Chemical Formula | C8H6FNO2 |
| Full Name | 1-Fluoro-4-(2-nitroethenyl)benzene |
| Molecular Weight | 167.14 g/mol |
| Synonyms | 1-(4-Fluorophenyl)-2-nitroethene, 1-Fluoro-4-(2-nitroethenyl)benzene, 1-(4-Fluorophenyl)-2-nitroethylene |
| Classification / Family | Fluorinated building blocks, Michael acceptors, APIs, Heterocycles, Antibiotics |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 99 °C – 102 °C |
| Appearance | Cream powder |
MSDS Documentation
 4-Fluoro-β-nitrostyrene MSDS Sheet
4-Fluoro-β-nitrostyrene MSDS Sheet
Literature and Reviews
- Augmentation of enantioselectivity by spatial aminocatalyst: synthesis of 2-alkyl/aryl-3-nitro-2H-chromenes by tandem oxa-Michael-Henry reaction, R. Mohanta et al., J. Org. Chem., 85(7), 4627–4636(2020);DOI: 10.1021/acs.joc.9b03366.
- N-Sulfinylpyrrolidine-containing ureas and thioureas as bifunctional organocatalysts, V. Poláčková et al., Beilstein J. Org. Chem., 17, 2629–2641(2021); DOI: 10.3762/bjoc.17.176.
- Comparisons of halogenated β-nitrostyrenes as antimicrobial agent, H. Cornell et al., Appl. Sci., 4, 380–389(2014); DOI: 10.3390/app4030380.
