4-Fluoroindole
CAS Number 387-43-9
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated indole
Used as a synthesis intermediate for dyes and APIs in application of drug discovery, OLEDs, DSSCs and bioimaging
Specifications | MSDS | Literature and Reviews
4-Fluoroindole (CAS number 387-43-9) is a mono-fluorinated indole, a benzene ring fused pyrrole heterocyclic compound. The derivatization of indole is versatile. Substitution of 1-position (on the nitrogen) is achieved with sodium hydride or potassium hydride making it a nucleophile. The 3-position can be activated with a Grignard reagent, namely methylmagnesium bromide or an organozinc compound. Position 2 of indole can be modified via directed ortho lithiation. After the expansion of chromophore, indole derivatives, triazatruxenes, are used as a donor unit in dye-sensitized solar cell with power conversion efficiency (PCE) of 13.6%.
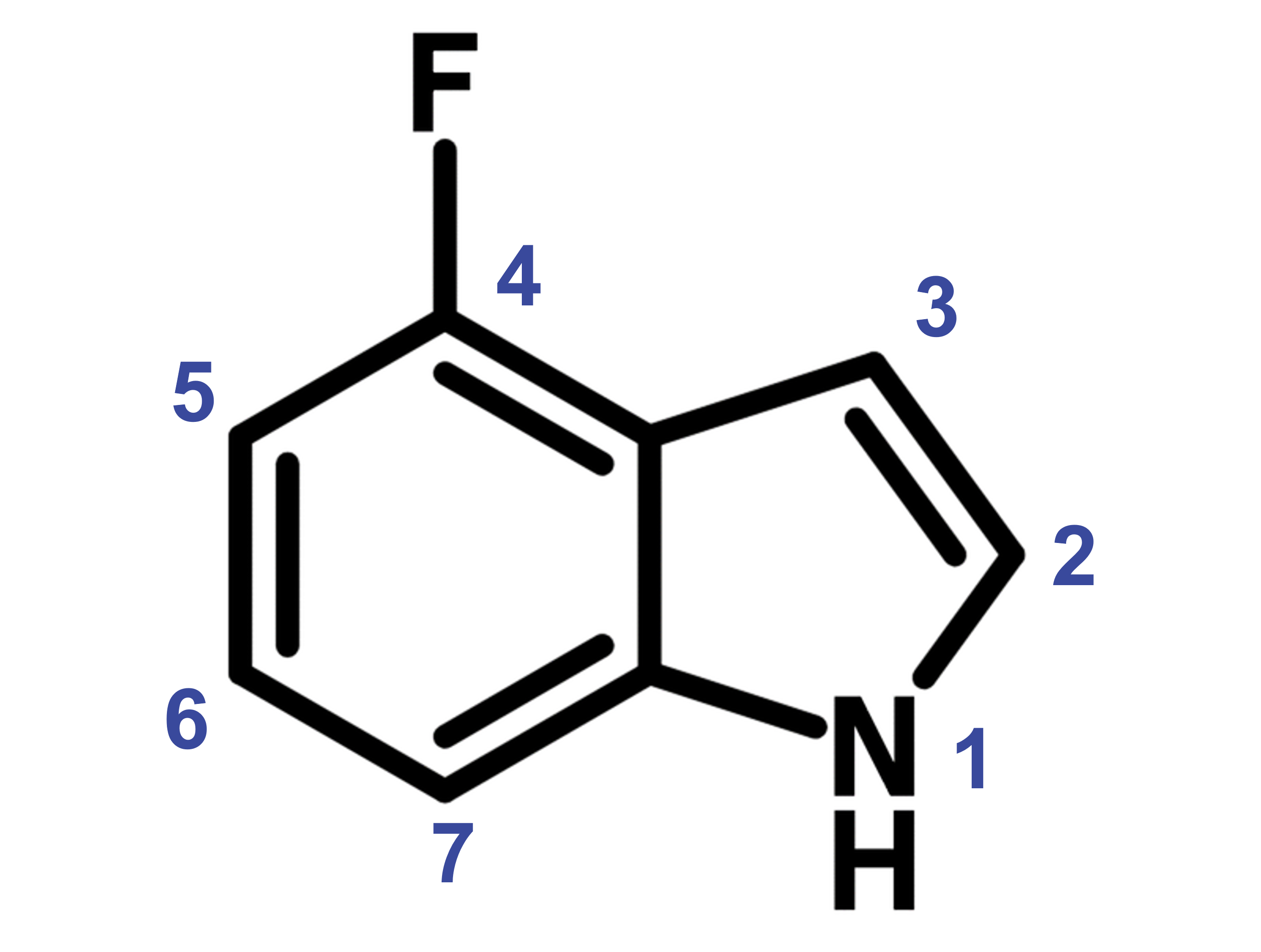
Indole is an intercellular signal molecule; hence it is a promising pharmaceutical molecular scaffold for active pharmaceutical ingredients (APIs). 4F-Indole improves the effectiveness of kanamycin (an aminoglycoside antibiotic) against Pseudomonas aeruginosa, a multi-drug resistant pathogen.
Combining the photophysio-properties and bioactivity, 4-fluoroindole is use in bioimaging, for instance monitoring cancer cells under anti-cancer treatments.
Multiple functional groups
For facile synthesis
Fluorinated indole building block
For drug discovery, OLEDs, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 387-43-9 |
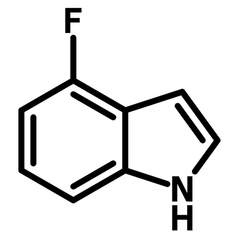
| Chemical Formula | C8H6FN |
| Full Name | 4-Fluoro-1H-indole |
| Molecular Weight | 135.14 g/mol |
| Synonyms | N/A |
| Classification / Family | Fluorinated building blocks, Heterocyclic building block, APIs, OLEDs, DSSCs, Bioimaging |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 30 °C – 32 °C |
| Appearance | White powder |
MSDS Documentation
Literature and Reviews
-
4F-Indole enhances the susceptibility of pseudomonas aeruginosa to aminoglycoside antibiotics, Q. Dou et al., Microbiol. Spectr., 11(2), e04519–e04522(2023); DOI: 10.1128/spectrum.04519-22.
-
Fine tuning of blue photoluminescence from indoles for device fabrication, J. Hwu et al., J. Mater. Chem., 19, 3084–3090(2009); DOI: 10.1039/b821246e.
-
Indole fused heterocycles as sensitizers in dye-sensitized solar cells: an overview, P. Nitha et al., Mater. Adv., 2, 6136(2021); DOI: 10.1039/d1ma00499a.

 4-Fluoroindole MSDS Sheet
4-Fluoroindole MSDS Sheet