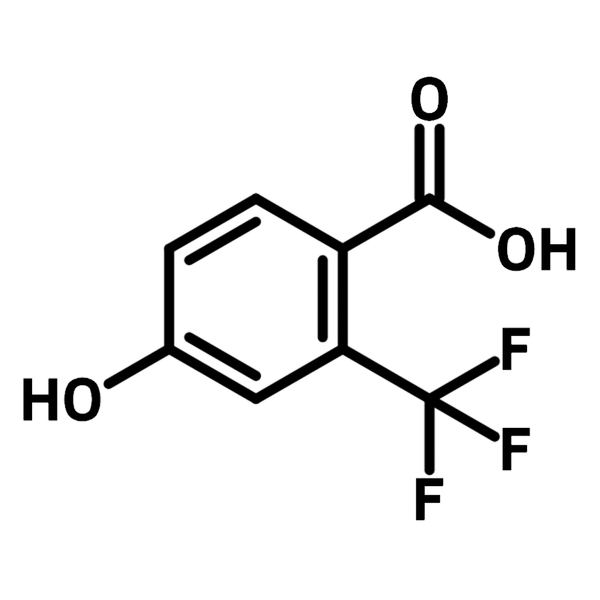
4-Hydroxy-2-(trifluoromethyl)benzoic acid
CAS Number 320-32-1
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, MonomersA trifluoromethylated benzoic acid building block
For synthesising functional polymers, liquid crystal and APIs
Specifications | MSDS | Literature and Reviews
4-Hydroxy-2-(trifluoromethyl)benzoic acid (CAS number 320-32-1) has hydroxyl, carboxylic acid and trifluoromethyl functional groups on the benzene ring. The carboxylic acid and hydroxyl groups are in the para-position, enabling it to synthesis linear molecules. Polyesters with segments of 4-hydroxy-2-(trifluoromethyl)benzoic acid self-assemble to form spherulite crystals for the fabrication of optical vortex beam generator. 4-Hydroxy-2-(trifluoromethyl)benzoic acid reacts with alkylated acyl chloride for the synthesis of smectic C liquid crystals.
Additionally, 4-hydroxy-2-(trifluoromethyl)benzoic acid can be attached to molecular scaffolds, such as benzofuran derivatives, through esterification and amide synthesis for producing active pharmaceutical ingredients (APIs) as antitubercular agents.
Multiple functional groups
For facile synthesis
Fluorinated benzoic acid building block
for drug discovery, liquid crystals, and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 320-32-1 |
| Chemical Formula | C8H5F3O3 |
| Full Name | 4-Hydroxy-2-(trifluoromethyl)benzoic acid |
| Molecular Weight | 206.12 g/mol |
| Synonyms | α,α,α-Trifluoro-4-hydroxy-o-toluic acid, 4-Carboxy-3-(trifluoromethyl)phenol |
| Classification / Family | Fluorinated building blocks, Benzoic acid building blocks, Phenol building blocks, Heterocycles, Liquid crystals, APIs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 159 °C – 161 °C |
| Appearance | White powder |
MSDS Documentation
 4-Hydroxy-2-(trifluoromethyl)benzoic acid MSDS Sheet
4-Hydroxy-2-(trifluoromethyl)benzoic acid MSDS Sheet
Literature and Reviews
- Design, in-silico study and biological evaluation of newly synthesized 3-chlorobenzofuran congeners as antitubercular agents, M. Abdullah et al., Arab. J. Chem., 14, 103034(2021); DOI: 10.1016/j.arabjc.2021.103034.
- Optical vortices enabled by structural vortices, Y. Liu et al., arXiv:2306.03359(2023); DOI: 10.48550/arXiv.2306.03359.
- Comprehensive review on coumarins: molecules of potential chemical and pharmacological interest, K. Kumar et al., J Chem Pharm Res, 7(9), 67–81(2015); ISSN : 0975-7384.
