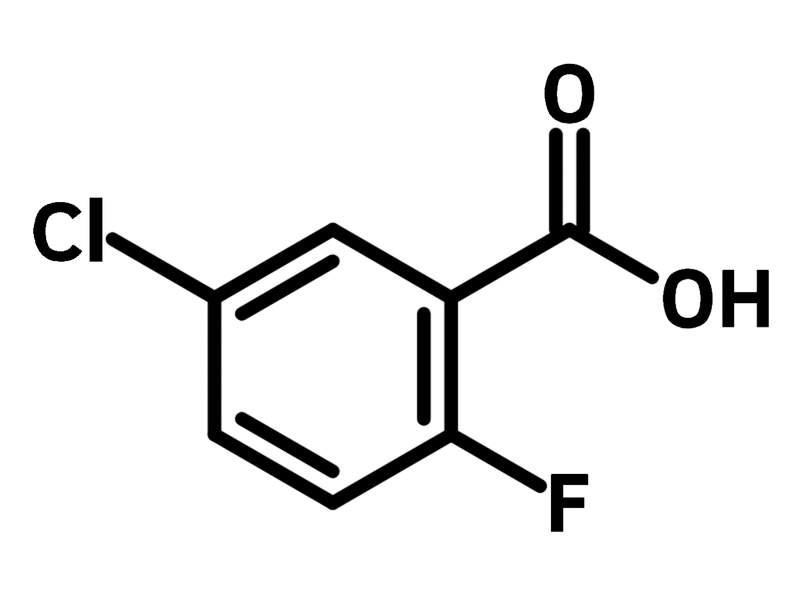
5-Chloro-2-fluorobenzoic acid
CAS Number 394-30-9
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksA dihalogenated benzoic acid building block
A synthesis intermediate for APIs and polymeric electrolyte membranes
Specifications | MSDS | Literature and Reviews
5-Chloro-2-fluorobenzoic acid (CAS number 394-30-9) is a derivative of benzoic acid substituted with fluoride and chloride groups. 5-Chloro-2-fluorobenzoic acid is used in the molecular design of pyrimidine-based Aurora kinase inhibitor. The chloride substituent in the target molecule enhances the binding affinity to Aurora A, promoting the degradation of oncoproteins.
5-Chloro-2-fluorobenzoic acid also finds its application in the synthesis of poly(phenylene ether)-based electrolyte membranes for proton exchange fuel cells. Firstly, 5-Chloro-2-fluorobenzoic acid is converted to benzoyl chloride using thionyl chloride, followed by Friedel-Crafts reaction with anisole. The polymer is then formed via the nickel-mediated homocoupling reaction. The polymeric electrolyte membrane exhibits high proton conductivity of 8.60 × 10−3 S cm−1 under 30% relative humidity at 80 °C.
Multiple functional groups
For facile synthesis
Fluorinated benzoic acid building block
For drug discovery, medicinal chemistry, and fuel cells
Low Cost
Competitively priced, high quality product
High purity
>98% High purity
General Information
| CAS Number | 394-30-9 |
| Chemical Formula | C7H4ClFO2 |
| Full Name | 5-Chloro-2-fluorobenzoic acid |
| Molecular Weight | 174.56 g/mol |
| Synonyms | 2-Fluoro-5-chlorobenzoic acid |
| Classification / Family | Fluorinated building blocks, Benzoic acid building blocks, APIs, Electrolyte membranes |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 152 °C – 157 °C |
| Appearance | White powder |
MSDS Documentation
 5-Chloro-2-fluorobenzoic acid MSDS Sheet
5-Chloro-2-fluorobenzoic acid MSDS Sheet
Literature and Reviews
- Polymer electrolyte membranes based on poly(phenylene ether)s with sulfonic acid via long alkyl side chains, X. Zhang et al., J. Mater. Chem. A, 1, 11389–11396 (2013); DOI: 10.1039/C3TA12026K.
- Discovery and synthesis of a pyrimidine-based aurora kinase inhibitor to reduce levels of MYC oncoproteins, Y.-H. Chi et al., J. Med. Chem. 2021, 64, 7312–7330 (2021); DOI: 10.1021/acs.jmedchem.0c01806.
- Discovery of novel small molecule dual inhibitors targeting toll-like receptor 7 and 8, R. Padilla-Salinas et al., J. Med. Chem., 62 (22), 10221–10244 (2019); DOI: 10.1021/acs.jmedchem.9b01201.
