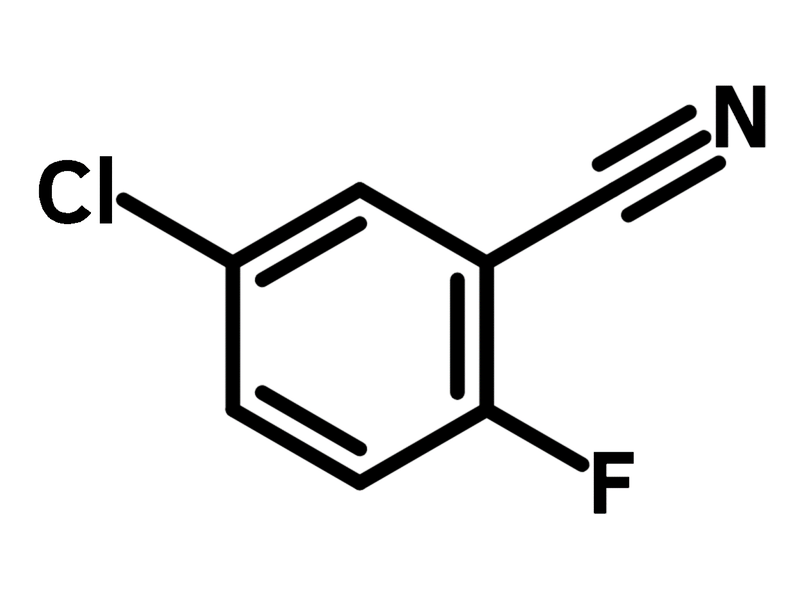
5-Chloro-2-fluorobenzonitrile
CAS Number 57381-34-7
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksA fluorinated benzonitrile building block
As a dihalogenated building block for the synthesis of APIs
Specifications | MSDS | Literature and Reviews
5-Chloro-2-fluorobenzonitrile (CAS number 57381-34-7) is a building block derived from benzonitrile, with fluoride next to the cyano and chloride opposite to the fluoride function group. 5-Chloro-2-fluorobenzonitrile can be used in the synthesis of aminobenzo[b]thiophene, in cooperation with ethyl mercaptoacetate under basic conditions. The resulting product is used as a kinase inhibitor and has enormous potential for further derivatization to achieve tailored binding affinity.
5-Chloro-2-fluorobenzonitrile is also employed in the preparation of aryl ether inhibitors of Bacillus anthracis enoyl–ACP reductase.
Multiple functional groups
For facile synthesis
Fluorinated benzonitrile building block
For drug discovery, medicinal chemistry, and organic synthesis
Low Cost
Competitively priced, high quality product
High purity
>98% High purity
General Information
| CAS Number | 57381-34-7 |
| Chemical Formula | C7H3ClFN |
| Full Name | 5-Chloro-2-fluorobenzonitrile |
| Molecular Weight | 155.56 g/mol |
| Synonyms | 5-Chloro-2-fluorocyanobenzene, 5-Chloro-2-fluorobenzyl cyanide |
| Classification / Family | Fluorinated building blocks, Benzonitrile building blocks, APIs, Bicyclic heterocycles |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 67 °C – 70 °C |
| Appearance | White Crystals |
MSDS Documentation
 5-Chloro-2-fluorobenzonitrile MSDS Sheet
5-Chloro-2-fluorobenzonitrile MSDS Sheet
Literature and Reviews
- Discovery of milvexian, a high-Affinity, orally bioavailable inhibitor of factor XIa in clinical studies for antithrombotic therapy, A. Dilger et al., J. Med. Chem., 65 (3), 1770–1785 (2022); DOI: 10.1021/acs.jmedchem.1c00613.
- Synthesis and structure-activity relationship studies of 2-(1,3,4-oxadiazole-2(3H)-thione)-3-amino-5-aryl-thieno[2,3-b]pyridines as inhibitors of DRAK2, P. Leonczak et al., ChemMedChem, 9 (11), 2587–2601 (2014); DOI: 10.1002/cmdc.201402234.
- Microwave-assisted synthesis of 3-aminobenzo[b]-thiophene scaffolds for the preparation of kinase inhibitors, M. Bagley et al., Org. Biomol. Chem., 13, 6814–6824 (2015); DOI: 10.1039/c5ob00819k.
