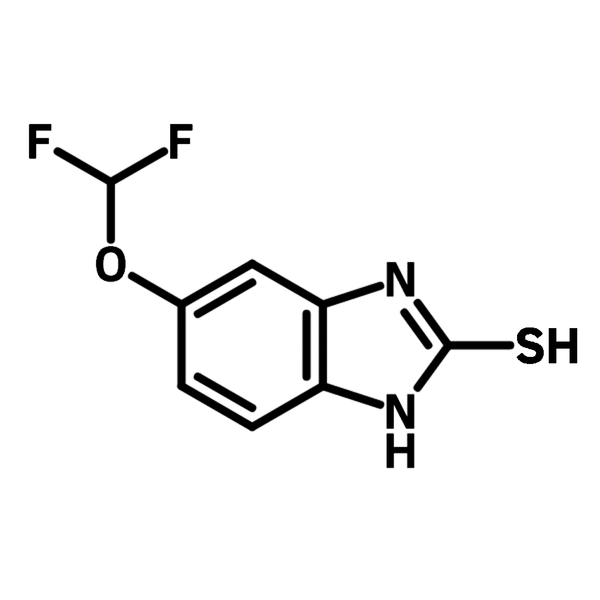
5-Difluoromethoxy-2-mercaptobenzimidazole
CAS Number 97963-62-7
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated mercaptobenzimidazole building block
Used as a synthesis intermediate for APIs in medicinal chemistry
Specifications | MSDS | Literature and Reviews
5-Difluoromethoxy-2-mercaptobenzimidazole (CAS number 97963-62-7) is the mercaptan derivative of benzimidazole, bearing a difluoromethoxy group substituted on the 5-position. 5-Difluoromethoxy-2-mercaptobenzimidazole is one of the key intermediates of pantoprazole, a proton pump inhibitor used for the treatment of stomach ulcers. Pantoprazole works by reducing the production of gastric acid and inactivating H+-ATPase in the stomach. The synthesis of pantoprazole starts with the coupling reaction of 5-difluoromethoxy-2-mercaptobenzimidazole with 2-chloromethyl-3,4-dimethoxypyridinium hydrochloride, followed by an oxidation reaction.
5-Difluoromethoxy-2-mercaptobenzimidazole is also utilised in the preparation of α‑glucosidase inhibitors for their efficacy in antidiabetic activity.
Multiple functional groups
For facile synthesis
Fluorinated building block
For drug discovery, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 97963-62-7 |
| Chemical Formula | C8H6F2N2OS |
| Full Name | 5-(Difluoromethoxy)-1,3-dihydro-2H-benzimidazole-2-thione |
| Molecular Weight | 216.21 g/mol |
| Synonyms | 5-(Difluoromethoxy)-2-benzimidazolethiol, Pantoprazole related compound C |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Benzimidazole building blocks, APIs |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 239 °C – 243 °C |
| Appearance | White powder |
MSDS Documentation
 5-Difluoromethoxy-2-mercaptobenzimidazole MSDS Sheet
5-Difluoromethoxy-2-mercaptobenzimidazole MSDS Sheet
Literature and Reviews
- Design, synthesis and biological evaluation of novel 3-hydroxypyridin-4(1H)-ones based hybrids as Pseudomonas aeruginosa biofilm inhibitors, J. Liu et al., Eur. J. Med. Chem., 259, 115665(2023); DOI: 10.1016/j.ejmech.2023.115665.
- Prospects to the formation and control of potential dimer impurity E of pantoprazole sodium sesquihydrate, A. Awasthi et al., J. Pharm. Anal., 9, 170–177(2019); DOI: 10.1016/j.jpha.2019.02.002.
- Mecaptobenzimidazole-based 1,3-thaizolidin-4-ones as antidiabetic agents: synthesis, in vitro α‑glucosidase inhibition activity, and molecular docking studies, S. Khan et al., ACS Omega, 7, 28041–28051(2022); DOI: 10.1021/acsomega.2c01969.
