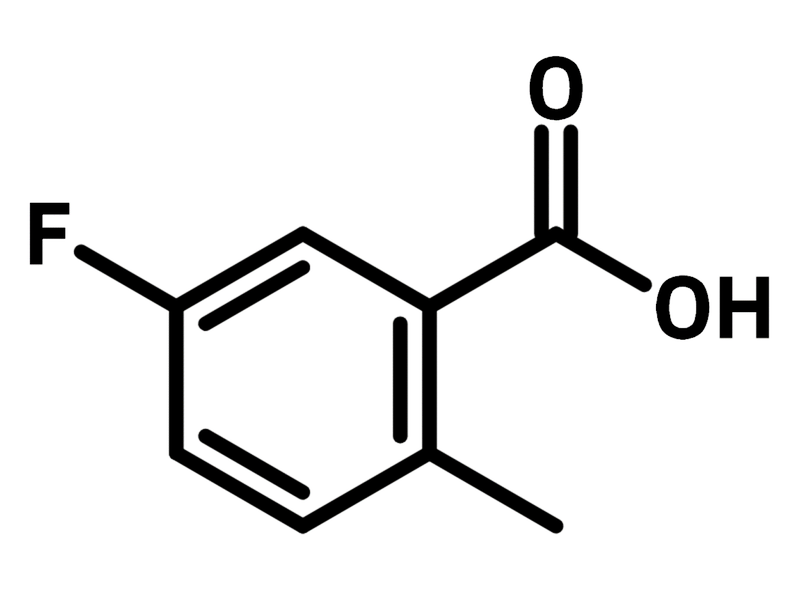
5-Fluoro-2-methylbenzoic acid
CAS Number 33184-16-6
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksA fluorinated benzoic acid building block
Utilised as a synthesis intermediate for APIs and as a substrate in organic synthesis
Specifications | MSDS | Literature and Reviews
5-Fluoro-2-methylbenzoic acid (CAS number 33184-16-6) is a fluorinated ortho-toluic acid. 5-Fluoro-2-methylbenzoic acid reacts with saturated ketones in a bimetallic Ir/Cu catalytic reaction to afford phthalide, a bicyclic heterocycle used in dyes and fungicides. Additionally, 5-fluoro-2-methylbenzoic acid is involved in the synthesis of benzamide derivatives as HIV-1 integrase inhibitors for antiviral treatment.
5-Fluoro-2-methylbenzoic acid is also utilized in the synthesis of 3-arylisoquinolinones with benzonitrile through a lithiation reaction. The resulting 3-arylisoquinolinones exhibit antiproliferative activity against cancer cells. These molecules bind to microtubules, suppress tubulin polymerization and induce apoptosis in cancer cells.
Multiple functional groups
For facile synthesis
Fluorinated benzoic acid building block
For drug discovery, medicinal chemistry, and biochemistry
Low Cost
Competitively priced, high quality product
High purity
>98% High purity
General Information
| CAS Number | 33184-16-6 |
| Chemical Formula | C8H7FO2 |
| Full Name | 5-Fluoro-2-methylbenzoic acid |
| Molecular Weight | 154.14 g/mol |
| Synonyms | 5-Fluoro-o-toluic acid, 2-Methyl-5-fluorobenzoic acid |
| Classification / Family | Fluorinated building blocks, Benzoic acid building blocks, APIs, Bicyclic heterocycles |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 130 °C – 132 °C |
| Appearance | White powder |
MSDS Documentation
 5-Fluoro-2-methylbenzoic acid MSDS Sheet
5-Fluoro-2-methylbenzoic acid MSDS Sheet
Literature and Reviews
- Iridium/copper-catalyzed oxidative C−H/O−H annulation of benzoic acids with saturated ketones for accessing 3-substituted phthalides, R. Wang et al., ChemCatChem, 12, 5907 (2020); DOI: 10.1002/cctc.202001214.
- Design of HIV-1 integrase inhibitors targeting the catalytic domain as well as its interaction with LEDGF/p75: a scaffold hopping approach using salicylate and catechol groups, X. Fan et al., Bioorg. Med. Chem., 19 (16), 4935–4952 (2011); DOI: 10.1016/j.bmc.2011.06.058.
- Validating and enabling phosphoglycerate dehydrogenase (PHGDH) as a target for fragment-based drug discovery in PHGDH-amplified breast cancer, J. Unterlass et al., Oncotarget, 9 (17), 13139–13153 (2018); DOI: 10.18632/oncotarget.11487.
