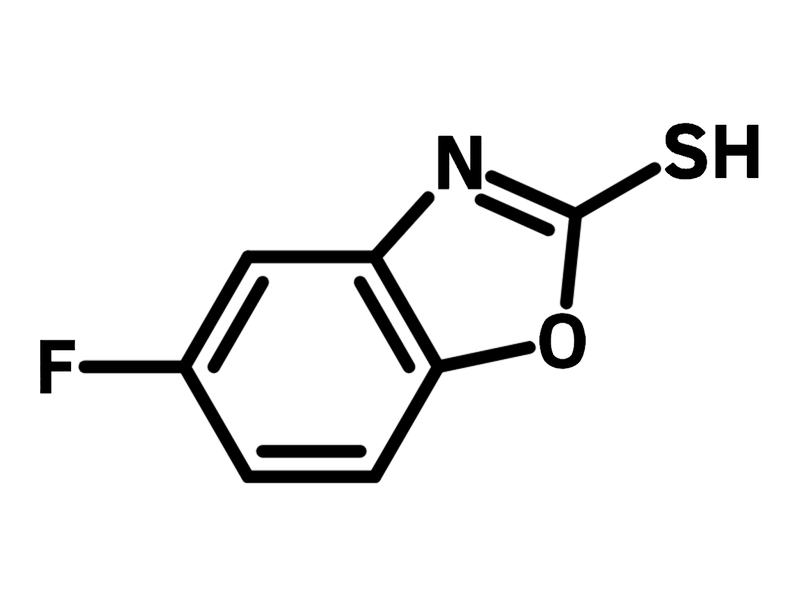
5-Fluorobenzoxazole-2-thiol
CAS Number 13451-78-0
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated benzoxazole building block
Serves as a synthesis intermediate for APIs
Specifications | MSDS | Literature and Reviews
5-Fluorobenzoxazole-2-thiol (CAS number 13451-78-0), also known as 5-fluoro-2-mercaptobenzoxazole, is a benzoxazole derivative featuring thiol and fluoride functional groups. Benzoxazole plays an important role in drug discovery, while the thiol group enables 5-fluorobenzoxazole-2-thiol to achieve desirable bioactivities via nucleophilic substitution. 5-Fluorobenzoxazole-2-thiol is modified with pyrrolidinamide for prodrugs against Mycobacterium tuberculosis, exhibiting a potency of 1.6 μM. Additionally, 5-Fluorobenzoxazole-2-thiol couples with phenylhydrazide derivatives, showing potential as antiglioma agents for anticancer drugs.
The thiol group in 5-fluorobenzoxazole-2-thiol can be converted to an amine through facile microwave- or visible light-assisted amination reactions.
Multiple functional groups
For facile synthesis
Fluorinated benzoxazole building block
For drug discovery, medicinal chemistry, and organic synthesis
Low Cost
Competitively priced, high quality product
High purity
>97% High purity
General Information
| CAS Number | 13451-78-0 |
| Chemical Formula | C7H4FNOS |
| Full Name | 5-Fluoro-1,3-benzoxazole-2(3H)-thione |
| Molecular Weight | 169.18 g/mol |
| Synonyms | 2-Mercapto-5-fluorobenzoxazole, 5-Fluoro-2-mercaptobenzoxazole, 5-Fluoro-2(3H)-benzoxazolethiene |
| Classification / Family | Fluorinated building blocks, Benzoxazole building blocks, Heterocyclic building blocks, APIs |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 238 °C – 240 °C |
| Appearance | Off-white powder |
MSDS Documentation
 5-Fluorobenzoxazole-2-thiol MSDS Sheet
5-Fluorobenzoxazole-2-thiol MSDS Sheet
Literature and Reviews
- Microwave-enhanced on-water amination of 2-mercaptobenzoxazoles to prepare 2-aminobenzoxazoles, T. Tankam et al., J. Org. Chem., 83 (19), 11936–11943 (2018); DOI: 10.1021/acs.joc.8b01824.
- Organocatalytic visible light enabled SNAr of heterocyclic thiols: a metal-free approach to 2-aminobenzoxazoles and 4-aminoquinazolines, E. Rattanagkool et al., J. Org. Chem., 82 (24), 13256–13262 (2017); DOI: 10.1021/acs.joc.7b02357.
- Substitution-modulated anticancer activity of half-sandwich ruthenium(II) complexes with heterocyclic ancillary ligands, R. Mitra et al., Eur. J. Inorg. Chem., 2014 (22), 3536–3546 (2014); DOI: 10.1002/ejic.201402205.
