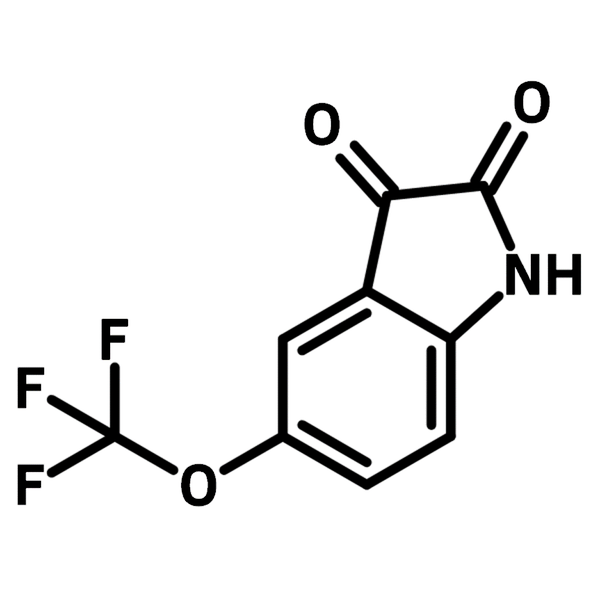
5-(Trifluoromethoxy)isatin
CAS Number 169037-23-4
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated heterocyclic building block
Used as a synthesis intermediate for organic and metal complexes in application of fluorescence dyes, OLEDs, and solar cells
Specifications | MSDS | Literature and Reviews
5-(Trifluoromethoxy)isatin (CAS number 169037-23-4), a derivative of indole, has two carbonyls at 2,3-position and a trifluoromethoxy at 5-position. In 5-(trifluoromethoxy)isatin, the carbonyl at 3-position is more reactive than the amide carbonyl at 2-position, thus it is readily functionalized under nucleophilic substitution. The nucleophilic substitution can also be enantioselective with the addition of chiral catalysts, with up to 92% ee (enantiomeric excess).
5-(Trifluoromethoxy)isatin is used to synthesize luminescent gold(I) complexes, displaying an emission at 400 nm. The isatin-gold complexes show cytotoxicity to cancer cells with half-maximal inhibitory concentration (IC50) as low as 0.28 μM. 5-(Trifluoromethoxy)isatin is also used to modify rhodamine in application of selectively detection of Cr3+. An isatin decorated fullerene (C60) has improved miscibility to P3HT in heterojunction organic solar cells.
Multiple functional groups
For facile synthesis
Fluorinated isatin building block
For drug discovery, solar cells, and OLEDs
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 169037-23-4 |
| Chemical Formula | C9H4F3NO3 |
| Full Name | 5-(Trifluoromethoxy)-1H-indole-2,3-dione |
| Molecular Weight | 231.13 g/mol |
| Synonyms | 5-(Trifluoromethoxy)-2,3-indolinedione |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Solar cells, OLEDs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 170 °C – 172 °C |
| Appearance | Orange to brown powder |
MSDS Documentation
 5-(Trifluoromethoxy)isatin MSDS Sheet
5-(Trifluoromethoxy)isatin MSDS Sheet
Literature and Reviews
- A novel Cr3+ fluorescence turn-on probe based on rhodamine and isatin framework, A. Dhara et al., J. Fluoresc., 25, 1921–1929 (2015); DOI: 10.1007/s10895-015-1684-0.
- Highly enantioselective construction of 3-hydroxy oxindole through a decarboxylative aldol addition of trifluoromethyl α-fluorinated gem-diol to N-benzyl isatins, I. Saidalimu et al., Angew. Chem. Int. Ed., 52, 5566–5570(2013); DOI: 10.1002/anie.201301443.
- Bioactive and luminescent indole and isatin based gold(I) derivatives, V. Fernández-Moreira et al., Dalton Trans., 48, 3098–3108(2019); DOI: 10.1039/C8DT00298C.
