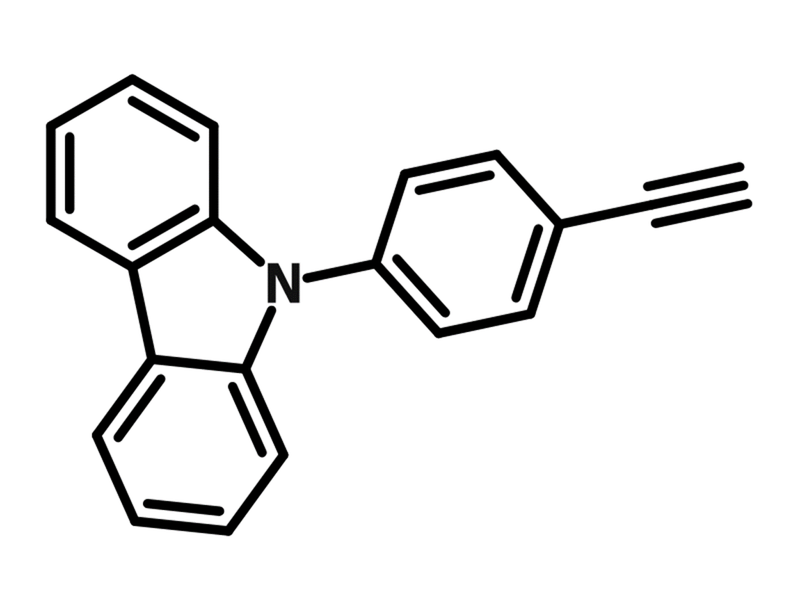
9-(4-Ethynylphenyl)carbazole
CAS Number 262861-81-4
Chemistry Building Blocks, COF Ligands, Heterocyclic Building Blocks, Porous Organic FrameworksCovalent Organic Frameworks (COFs) Carbazole Ligand
Used in the synthesis of macromolecules as photoluminescent and supercapacitor materials
Overview | Product Information | MSDS | Related Products
9-(4-Ethynylphenyl)carbazole (CAS number 262861-81-4) is a carbazole derivative functionalized with an ethynylphenyl group. Owing to the ethynyl group, 9-(4-ethynylphenyl)carbazole can undergo the Sonogashira coupling reaction and click reaction to form semiconducting macromolecules. Additionally, 9-(4-ethynylphenyl)carbazole can form oligomers through oxidation reactions with FeCl3.
9-(4-Ethynylphenyl)carbazole is employed in the synthesis of carbazole-functionalised corannulene-viologen ambipolar polymers that are used in electrochromic supercapacitors. The resulting polymer exhibits high optical contrast and specific capacitance of 394 F/g. 9-(4-Ethynylphenyl)carbazole is attached to naphthalene and perylene diimides, forming D-π-A-π-D n-type semicondutors with electron mobility of up to 0.3 cm2/V·s.
MOF and COF Ligands
Carbazole ligand for cross-linked COF/MOF networks
Facile Reactions
Ethynyl possesses excellent reactivity
High Purity
> 97% pure
Worldwide Shipping
Quick and reliable shipping
General Information
| CAS Number | 262861-81-4 |
|---|---|
| Chemical Formula | C20H13N |
| Full Name | 9-(4-Ethynylphenyl)-9H-carbazole |
| Molecular Weight | 267.33 g/mol |
| Synonyms | N/A |
| Classification or Family | Carbazoles, Alkynes, Photoluminescent, Semiconductors, Supercapacitors, Electrochemistry |
Chemical Structure
Product Details
| Purity | > 97% |
|---|---|
| Melting Point | Tm = 104 – 108 °C |
| Appearance | Yellow powder |
MSDS Documentation
9-(4-Ethynylphenyl)carbazole MSDS Sheet
References
- Synthesis of luminescent 2-7 disubstituted silafluorenes with alkynyl-carbazole, -phenanthrene, and -benzaldehyde substituents, S. Germann et al., J. Organomet. Chem., 927, 121514 (2020); DOI: 10.1016/j.jorganchem.2020.121514.
- Synthesis of fused chalcogenophenocarbazoles: towards dual emission resulting from hybridized local and charge-transfer states, A. Petrenko et al., New J. Chem., 44, 3903–3911 (2020); DOI: 10.1039/C9NJ06211D.
- Extended π-conjugation and structural planarity effects of symmetrical D-π-A-π-D naphthalene and perylene diimide semiconductors on n-type electrical properties, S. Gámez-Valenzuela et al., Chem. Eur. J., 29 (46), e202301639 (2023); DOI: 10.1002/chem.202301639.
Related Products
We stock a wide range of porous organic frameworks available to purchase online. Please contact us if you cannot find what you are looking for.