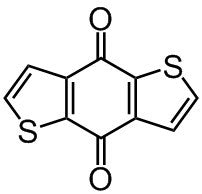
Benzo[1,2-b:4,5-b']dithiophene-4,8-dione
CAS Number 32281-36-0
Chemistry Building Blocks, Heterocyclic Building Blocks, Materials, MonomersBenzo[1,2-b:4,5-b']dithiophene-4,8-dione, for the application of OFETs and OPVs
High purity (>99%) and available online for fast, secure dispatch
Specifications | MSDS | Literature and Reviews
Benzo[1,2-b:4,5-b’]dithiophene, CAS number 32281-36-0, is a planar symmetrical molecular structure of the thiophene derivative, enabling a better π-π stacking and good electron delocalization that encourages charge transport. In recent years, it has been intensively studied for the application of OFETs and OPVs. The incorporation of a low-band-gap unit into the benzo[1,2-b:4,5-b’]dithiophene unit could potentially result in a red-shifted absorption due to its electron-rich properties. Benzo[1,2-b:4,5-b’]dithiophene can be chemically modified to fine-tune its chemical structure and electron properties (e.g. band gap), energy levels, and charge mobility of the small molecule/polymers of interest (at a molecular level).
Benzo[1,2-b:4,5-b’]dithiophene (BDT) backboned polymers or small molecules thus provides a relatively better device performance. The following is a good example of a finely tuned structure being synthesised with Benzo[1,2-b:4,5-b’]dithiophene as a starting material.
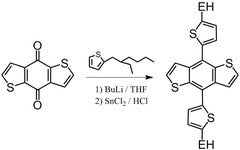
A synthesis precusor
For benzodithiophene backboned polymers
Benzodithiophenedione building block
For semiconductors, OFETs, and solar cells
Worldwide shipping
Quick and reliable shipping
High purity
>99% High purity
General Information
| CAS Number |
32281-36-0 |
| Chemical Formula | C10H4O2S2 |
| Molecular Weight | 220.26 g/mol |
| Synonyms | Benzo[1,2-b:4,5-b']bisthiophene-4,8-dione Thieno[2,3-f][1]benzothiophene-4,8-dione |
| Classification / Family | Thiophenes, Monomers, Building blocks, Carbazoles, Heterocycles, Polymer synthesis, Semiconductor synthesis, Intermediates for high performance, Organic Photovoltaics |
Chemical Structure
Product Details
| Purity | >99% |
| Melting Point | 260 °C - 263 °C |
| Appearance | Yellow powder |
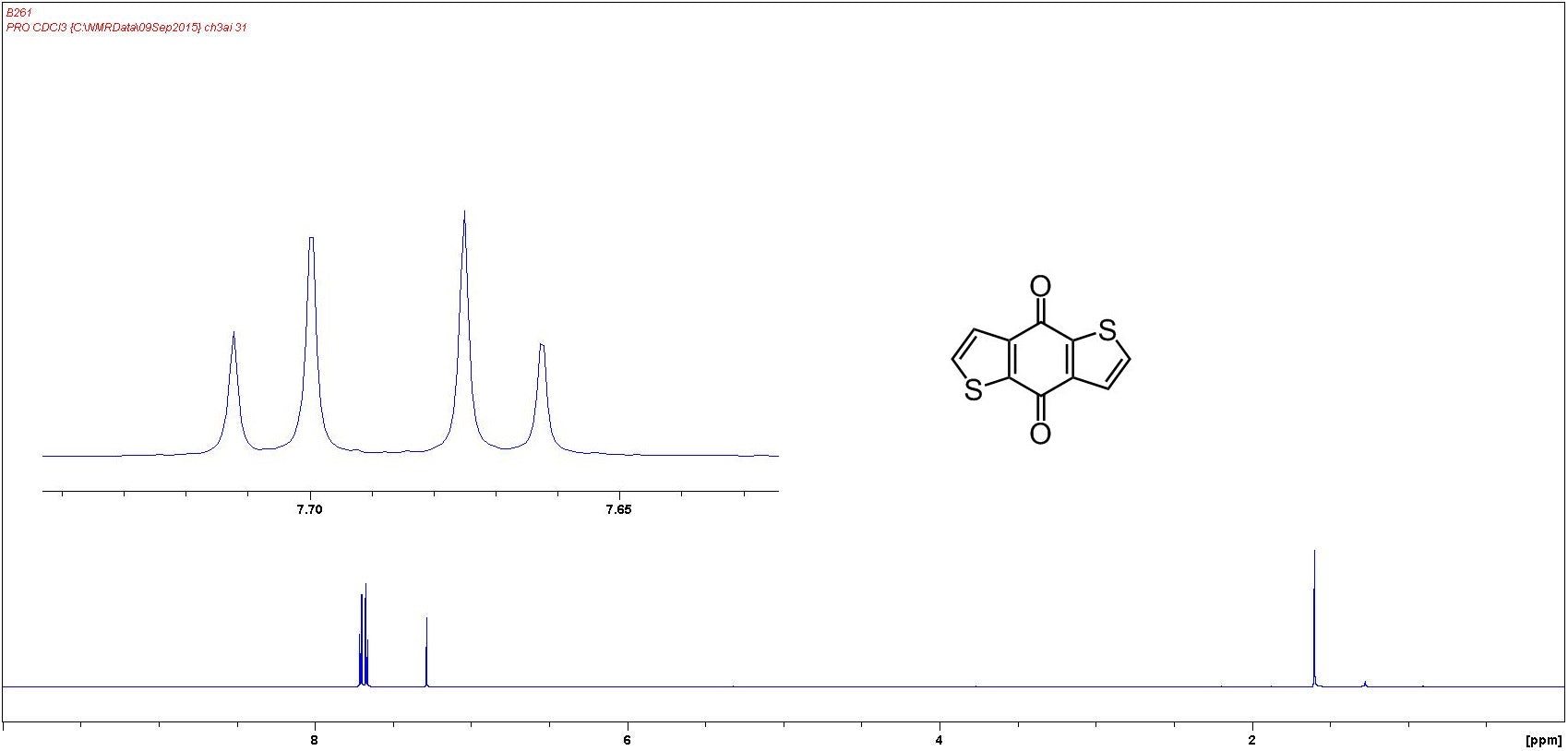
NMR Characterisation
MSDS Documentation
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione MSDS](https://www.ossila.com/cdn/shop/files/msds-sheets.jpg?width=60&height=60) Benzo[1,2-b:4,5-b']dithiophene-4,8-dione MSDS sheet
Benzo[1,2-b:4,5-b']dithiophene-4,8-dione MSDS sheet
Literature and Reviews
- Utilizing alkoxyphenyl substituents for side-chain engineering of efficient benzo[1,2-b:4,5-b′]dithiophene-based small molecule organic solar cells, Z. Du, et al., Phys. Chem. Chem. Phys., 17, 17391-17398 (2015)
- Thieno[3,2‑b]thiophene-Substituted Benzo[1,2‑b:4,5‑b′]dithiophene as a Promising Building Block for Low Bandgap Semiconducting Polymers for High-Performance Single and Tandem Organic Photovoltaic Cells, J-H. Kim, et al., Chem. Mater., 26, 1234−1242 (2014)
- New Benzo[1,2-b:4,5-b’]dithiophene-Based Small Molecules Containing Alkoxyphenyl Side Chains for High Efficiency Solution-Processed Organic Solar Cells, Z. Du, et al., ChemSusChem, 7, 3319 – 3327 (2014)
![Benzo[1,2-b:4,5-b']dithiophene-4,8-dione CAS 32281-36-0](http://www.ossila.com/cdn/shop/files/benzo-dithiophene-dione.jpg?v=1718724889&width=240)