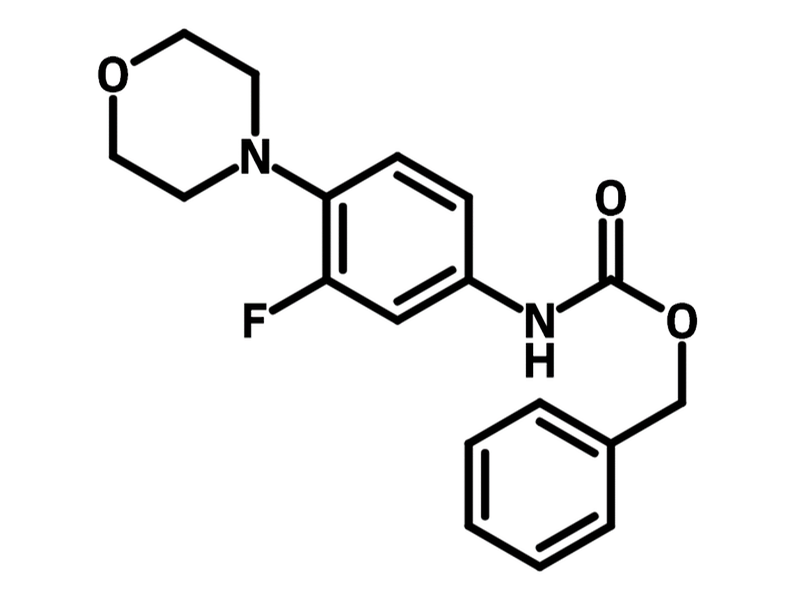
Benzyl (3-Fluoro-4-morpholinophenyl)carbamate
CAS Number 168828-81-7
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated heterocyclic building block
Used as a synthesis intermediate for APIs and non-linear optical materials
Specifications | MSDS | Literature and Reviews
Benzyl (3-Fluoro-4-morpholinophenyl)carbamate (CAS number 168828-81-7) is a fluorinated heterocyclic compound, bearing a morpholine (a 6-membred heterocycle), a fluorobenzene, a carbamate linker and a benzyl group. Benzyl (3-Fluoro-4-morpholinophenyl)carbamate is one of the intermediates for synthesising linezolid, an antibiotic for treatment of Gram-positive bacteria that is resistant to other antibiotics. To synthesise linezolid, benzyl (3-Fluoro-4-morpholinophenyl)carbamate reacts with bromomethyloxirane forming an oxazolidone moiety. The bromide is subsequently replaced by acetamide to complete the molecular formation. Other than linezolid, benzyl (3-Fluoro-4-morpholinophenyl)carbamate is also used to synthesise glucose uptake inhibitor for supressing the growth of cancer cells.
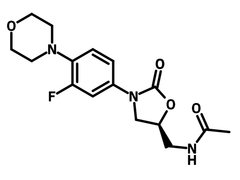
A theoretical study has revealed that benzyl (3-Fluoro-4-morpholinophenyl)carbamate has a large first-order hyperpolarizability for potential use in non-linear optical applications.
Multiple functional groups
For facile synthesis
Fluorinated morpholinophenyl building block
For drug discovery, medicinal chemistry, and photonics
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 168828-81-7 |
| Chemical Formula | C18H19FN2O3 |
| Full Name | Benzyl (3-Fluoro-4-morpholinophenyl)carbamate |
| Molecular Weight | 303.36 g/mol |
| Synonyms | (3-Fluoro-4-morpholinophenyl)carbamic acid benzyl ester |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, APIs, Photonics |
Chemical Structure
Product Details
| Purity | >98% |
| Melting Point | Tm = 125 °C |
| Appearance | Off-white fluffy powder |
MSDS Documentation
 Benzyl (3-Fluoro-4-morpholinophenyl)carbamate MSDS Sheet
Benzyl (3-Fluoro-4-morpholinophenyl)carbamate MSDS Sheet
Literature and Reviews
-
A novel and expeditious synthesis of oxazolidinone drugs linezolid and eperezolid, R. Siddaraj et al., Eur. J. Chem., 9(4), 353–359(2018); DOI: 10.5155/eurjchem.9.4.353-359.1783.
-
Inhibition of glucose transporters and glutaminase synergistically impairs tumor cell growth, E. Reckzeh et al., Cell Chem. Biol., 26, 1214–1228(2019); DOI: 10.1016/j.chembiol.2019.06.005.
-
The role of oxazolidine derivatives in the treatment of infectious and chronic diseases, J. Branco-Junior et al., Curr. Bioact. Compd., 13(4), 292–304(2017); DOI: 10.2174/1573407213666161214162149.

