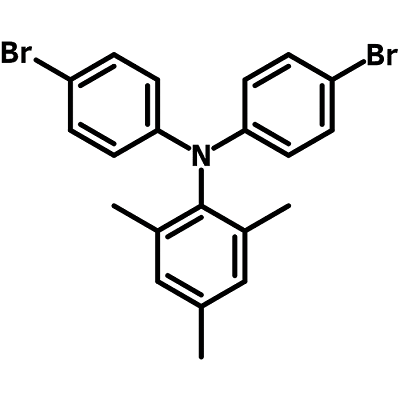
N,N-Bis(4-bromophenyl)-2,4,6-trimethylaniline
CAS Number 663943-27-9
Chemistry Building Blocks, Dibromo Monomers, Materials, Monomers, Non-Heterocyclic Building BlocksHigh-purity N,N-Bis(4-bromophenyl)-2,4,6-trimethylaniline
A convenient intermediate for the synthesis of polytriarylamines in application of DSSC, OLEDs and perovskite solar cells.
Specifications | MSDS | Literature and Reviews
N,N-Bis(4-bromophenyl)-2,4,6-trimethylaniline (CAS number 663943-27-9) has a triarylamine structure with one 2,4,6-methylated phenyl and two 4-brominated phenyl rings. The two bromo functional groups at the end of each benzene rings enable it to extend its conjugation via Suzuki, Yamamoto or Stille coupling reactions.
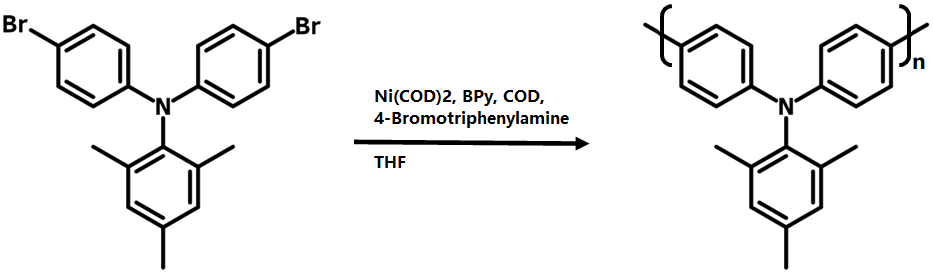
N,N-Bis(4-bromophenyl)-2,4,6-trimethylaniline is a convenient intermediate for the synthesis of polytriarylamines (PTAAs) via Yamamoto polycondensation. PTAAs are electron rich thus commonly used as electron transport layer for perovskite solar cells and OLED devices. PTAAs can normally be deposited by solution processing at lower temperature, significantly reducing possible thermal damage to the active layer.
N,N-Bis(4-bromophenyl)-2,4,6-trimethylaniline is prepared from the reaction of 2,4,6-trimethylaniline with 1-bromo-4-iodobenzene (Buchwald-Hartwig amination) in the presence of 1,1′-ferrocenediyl-bis(diphenylphosphine) (dppf) and sodium tert-butoxide in toluene.
General Information
| CAS Number | 663943-27-9 |
| Chemical Formula | C21H19Br2N |
| Full Name | N,N-Bis(4-bromophenyl)-2,4,6-trimethylaniline |
| Molecular Weight | 445.19 g/mol |
| Synonyms | N,N-Bis(4-bromophenyl)-2,4,6-trimethylaniline |
| Classification / Family | PTAA, Semiconductor synthesis intermediates, Hole transport layer materials, Perovskite solar cells |
Chemical Structure
Product Details
| Purity | >98% (NMR) |
| Melting Point | N/A |
| Appearance | Yellowish white powder/crystals |
MSDS Documentation
 N,N-Bis(4-bromophenyl)-2,4,6-trimethylaniline MSDS Sheet
N,N-Bis(4-bromophenyl)-2,4,6-trimethylaniline MSDS Sheet
Pricing
| Batch | Quantity | Price |
| B1101-1g | 1 g | £140 |
| B1101-5g | 5 g | £560 |
| B1101-10g | 10 g | £900 |
Literature and Reviews
-
Suzuki polycondensation for the synthesis of polytriarylamines: A method to improve hole-transport material performance in perovskite solar cells, M. Tepliakova et al., Tetrahedron lett., 61 (38), 152317 (2020); DOI: 10.1016/j.tetlet.2020.152317.
-
Donor–π–Acceptor Polymer with Alternating Triarylborane and Triphenylamine Moieties, H. Li et al., Macromol. Rapid Commun., 31, 915 (2010); DOI: 10.1002/marc.200900932.
- Methoxy-Functionalized Triarylamine-Based Hole-Transporting Polymers for Highly Efficient and Stable Perovskite Solar Cells, Y. Kim et al., ACS Energy Lett., 5 (10), 3304–3313 (2020).
