D18-Cl, highly efficient polymer semiconductor for NF-PSCs
Deeper HOMO and LUMO levels than D18
Specifications | MSDS | Literature and Reviews
D18-Cl, a chlorinated version of D18, is another highly efficient polymer semiconductor that is demonstrating over 18% PCE in non-fullerene polymer solar cells (NF-PSCs).
When compared with D18, which has fluorinated thiophene side chains, shows both deeper HOMO and LUMO energy levels. A device efficiency of 18.12% and certified efficiency of 17.6% have been achieved using D18-Cl as the electron donor and N3 as an acceptor in a single junction non-fullerene polymer solar cell (NF-PSC) [1]. Further improvement of device performance to an outstanding PCE of 18.69% was obtained while a second acceptor PCBM was used in the active layer [2]. PCE of 20.8% was achieved by using D18-Cl as the polymer donor and BTP-4F-P2EH as the NFA acceptor by engaging the same layer-by-layer deposition method [3].
Narrow bandgap copolymer
For high efficient OPV applications
Luminosyn™
High purity, batch-specific GPC data, available larger batch orders and higher molecular weight
Worldwide shipping
Quick and reliable shipping
Green energy materials
Processable in non-halogenated solvents
Luminosyn™ D18-Cl
Luminosyn™ D18-Cl is now available.
High purity
D18-Cl is purified via Soxhlet extraction with acetone, hexane, and chlorobenzene under an argon atmosphere
Large quantity orders
Plan your experiments with polymers from the same batch
General Information
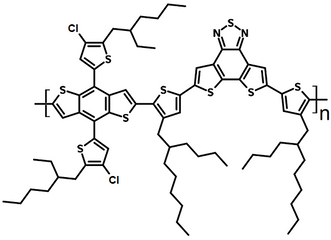
| Full name | Poly[(2,6-(4,8-bis(5-(2-ethylhexyl)-4-chlorothiophen-2-yl)-benzo[1,2-b:4,5-b']dithiophene))-alt-5,5'-(5,8-bis(4-(2-butyloctyl)thiophen-2-yl)dithieno[3',2':3,4;2'',3'':5,6]benzo[1,2-c][1,2,5]thiadiazole)] |
| Synonyms | PBDBTCl-DTBT |
| Chemical formula | (C76H92Cl2N2S7)n |
| CAS number | n.a. |
| HOMO / LUMO | HOMO = -5.56 eV, LUMO = -2.78 eV [1] |
| Soluble in | THF, o-xylene, chloroform, chlorobenzene and dichlorobenzene |
| Recommended Processing Solvents at 10mg/ml | THF (8mg/ml), o-xylene (8mg/ml), chloroform |
| Classification / Family | Organic semiconducting materials, Medium bandgap polymers, Organic photovoltaics, Polymer solar cells, Perovskite solar cells, Hole-transport layer materials, NF-PSCs, All-polymer solar cells (all-PSCs). |
Batch Details
| Batch | Mw | Mn | PDI | Stock Info |
| M2281A1 | 31,139 | 16,456 | 1.89 | In stock |
Chemical Structure
Device Structure(s)
Device structure 1: ITO/PEDOT:PSS/D18-Cl:N3 (1:1.4)/PDIN/Ag
| Thickness (nm) | VOC (V) | JSC (mA cm-2) | FF (%) | PCE (%) |
| 108 | 0.859 | 27.85 | 75.7 | 18.13 |
Device structure 2: ITO/PEDOT:PSS/D18-Cl:N3:PCBM (1:1.4:0.1)/PDIN/Ag
| Thickness (nm) | VOC (V) | JSC (mA cm-2) | FF (%) | PCE (%) |
| 114 | 0.849 | 28.22 | 78 | 18.69 |
MSDS Documentation
Literature and Reviews
- A chlorinated copolymer donor demonstrates a 18.13% power conversion efficiency, J. Qin et al., J. Semicond., 42 (1), 010501 (2021); DOI: 10.1088/1674-4926/42/1/010501.
-
18.69% PCE from organic solar cells, K. Jin et al., J. Semicond., 42(6), 060502 (2021); DOI: 10.1088/1674-4926/42/6/060502.
-
Achieving 20.8% organic solar cells via additive-assisted layer-by-layer fabrication with bulk p-i-n structure and improved optical management, L. Zhu et al., Joule (2024); DOI: 10.1016/j.joule.2024.08.001.
-
Approaching 18% efficiency of ternary organic photovoltaics with wide bandgap polymer donor and well compatible Y6 : Y6-1O as acceptor, X. Ma et al., Natl. Sci. Rev. 8, nwaa305 (2021); DOI: 10.1093/nsr/nwaa305.


 D18-Cl MSDS sheet
D18-Cl MSDS sheet