2,8-Dibromodibenzothiophene
CAS Number 31574-87-5
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials, MonomersHigh-Quality 2,8-Dibromodibenzothiophene
A useful building block for the further synthesis of small molecules, oligomers and polymers either as host for phosphorescent dyes, electron transport layer or active layer materials for OLEDs, OFETs and OPVs.
Specifications | MSDS | Literature and Reviews
2,8-Dibromodibenzothiophene (CAS number 31574-87-5) is a double brominated derivative of dibenzothiophene which structurally can be considered as a biphenyl further bridged with a sulfur atom. The numbering of dibenzothiophene ring is somewhat confusing when it is compared with the numbering of carbazole.
2,8-Dibromodibenzothiophene was synthesised from dibenzothiophene with bromine in chloroform. Further oxidation of 2,8-dibromodibenzothiophene with dihydrogen peroxide In acetic acid gives 2,8-dibromodibenzothiophene- 5,5-dioxide. Solution-processed OFETs using the syn-TBBT, prepared from 2,8-dibromodibenzothiophene as the starting material, show high hole mobilities of up to 10.1 cm2 V-1s-1. Water-soluble π-conjugated small molecules based on dibenzothiophene-S,S-dioxide and bispyridinium salts, FSOPyCl and FSOmiCl, show large planarity, good thermal stability, excellent electron affinity and high fluorescence efficiency. Ambipolar phosphorescent host and electron transport layer material 2,8-Bis(diphenyl-phosphoryl)-dibenzo[b,d]thiophene (PPT), also a derivate of 2,8-dibromodibenzothiophene, yields a power efficiency of 30 lm W-1 and current efficiency of 42 cd/A while hosting FIrPic as blue emitting OLEDs with the following device structure:
ITO/TAPC (40 nm)/TCTA (2 nm)/26DCzPPy:TCTA:FIrPic (0.4:0.4:0.2) (5 nm)/26DCzPPy:PPT:FIrpic (0.4:0.4:0.2) (5 nm)/3TPYMB (55 nm)/CsF (2 nm)/Al (180 nm)
Dibenzothiophene building block
for the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Capped with bromides
for facile coupling reactions
High purity
>99% Purity
General Information
| CAS Number | 31574-87-5 |
| Chemical Formula | C12H6Br2S |
| Full Name | 2,8-Dibromodibenzo[b,d]thiophene |
| Molecular Weight | 342.05 g/mol |
| Synonyms |
DBDBT 2,8-Dibromo Dibenzothiophene |
| Classification / Family | Dibromodibenzothiophene, semiconductor synthesis intermediates, low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
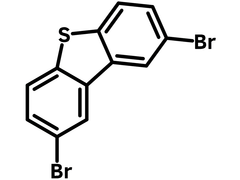
Product Details
| Purity | >99% (HPLC) |
| Melting point | Tm = 226 °C |
| Appearance | White to off-white powder/crystals |
MSDS Documentation
 2,8-Dibromodibenzothiophene MSDS sheet
2,8-Dibromodibenzothiophene MSDS sheet
Literature and Reviews
-
High-Mobility Regioisomeric Thieno[f,f′]bis[1]benzothiophenes: Remarkable Effect of Syn/Anti Thiophene Configuration on Optoelectronic Properties, Self-Organization, and Charge-Transport Functions in Organic Transistors, T. Oyama et al., Adv. Funct. Mater., 4 (1), 1700390 (2018); DOI: 10.1002/aelm.201700390.
-
Dipolar Dibenzothiophene S,S-Dioxide Derivatives Containing Diarylamine: Materials for Single-Layer Organic Light-Emitting Devices, T. Huang et al., Adv. Mater., 18, 602–606 (2006); DOI: 10.1002/adma.200502078.
-
Organic electroluminescent derivatives containing dibenzothiophene and diarylamine segments, T. Huang et al., J. Mater. Chem., 15, 3233–3240 (2005); DOI: 10.1039/b507210.
