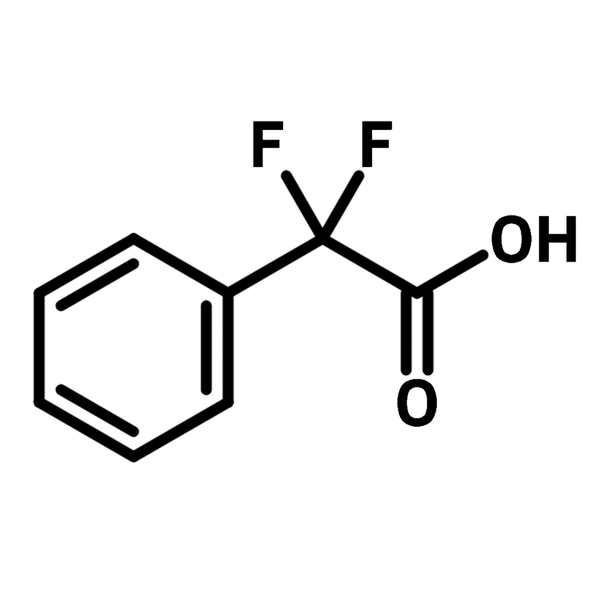
α,α-Difluorophenylacetic acid
CAS Number 360-03-2
Chemistry Building Blocks, Fluorinated Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksA fluorinated phenylacetic acid building block
Used as a difluoromethylation reagent for pharmaceutical and agrochemical ingredients
Specifications | MSDS | Literature and Reviews
α,α-Difluorophenylacetic acid (CAS number 360-03-2) is a phenyl acetic acid with two fluorides on the benzylic carbon. The inductive effect of the difluoromethyl group increases the acidity of α,α-difluorophenylacetic acid. α,α-Difluorophenylacetic acid is primarily used to introduce benzylic difluoromethylene group to molecular scaffolds through radical decarboxylative reaction. The reaction begins with an oxidative decarboxylation with persulfate forming a difluorobenzyl radical that is stabilised by the benzene and fluorine groups. The addition is carried out by the attack of the radical species to the alkenes or imines. The decarboxylative reaction is versatile and can even be performed in an aqueous solution.
Multiple functional groups
For facile synthesis
Fluorinated phenylacetic acid building block
For organic synthesis, medicinal chemistry and biochemistry research
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
General Information
| CAS Number | 360-03-2 |
| Chemical Formula | C8H6F2O2 |
| Full Name | α,α-Difluorophenylacetic acid |
| Molecular Weight | 172.13 g/mol |
| Synonyms | 2,2-Difluoro-2-phenylacetic acid |
| Classification / Family | Fluorinated building block, Decarboxylative reactions, APIs |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | Tm = 65 °C – 75 °C |
| Appearance | White crystals |
MSDS Documentation
 α,α-Difluorophenylacetic acid MSDS Sheet
α,α-Difluorophenylacetic acid MSDS Sheet
Literature and Reviews
- Photoinitiated decarboxylative C3-difluoroarylmethylation of quinoxalin-2(1H)-ones with potassium 2,2-difluoro-2-arylacetates in water, Y. Gao et al., RSC Adv., 10, 10559(2020); DOI: 10.1039/d0ra02059a.
- Insight into the mechanism of phenylacetate decarboxylase (PhdB), a toluene-producing glycyl radical enzyme, A. Rodrigues et al., ChemBioChem, 21, 663–671(2020); DOI: 10.1002/cbic.201900560.
- Synthesis of difluoroarymethyl-substituted benzimidazo[2,1-a]isoquinolin-6(5H)-ones under mild conditions, K. Sui et al., ACS Omega, 8, 7517–7528(2023); DOI: 10.1021/acsomega.2c06689.
