DPP-DTT, PDPP2T-TT-OD
CAS Number 1260685-66-2 / 1444870-74-9
Interface Polymers, Luminosyn™ Polymers, Materials, OFET & OLED Polymer Materials, OPV Polymers, Semiconducting PolymersDPP-DTT, high quality and high purity semiconducting polymer
High performance p-type polymer and donor material for BHJ photovoltaics
Overview | Specifications | Pricing and Options | MSDS | Literature and Reviews
DPP-DTT is a high mobility p-type polymer, suitable for OFET and sensing and photovoltaic applications.
DPP-DTT from Ossila was used in the high-impact paper (IF 18.81), Stretchable Mesh-Patterned Organic Semiconducting Thin Films on Creased Elastomeric Substrates, S. Kim et al., Adv. Funct. Mater., 2010870 (2021); DOI: 10.1002/adfm.202010870.
Luminosyn™ DPP-DTT
Luminosyn™ DPP-DTT (also referred to as PDPP2T-TT-OD) is now available.
High molecular weight
Higher molecular weight offers higher charge mobility
High purity
DPP-DTT is purified via Soxhlet extraction with methanol, hexane and chlorobenzene under an argon atmosphere
Batch-specific GPC data
Have confidence in what you are ordering; batch-specific GPC data for your thesis or publications
Large quantity orders
Plan your experiments with confidence with polymers from the same batch
General Information
| CAS number | 1260685-66-2 (1444870-74-9) |
| Chemical formula | (C60H88N2O2S4)n |
| HOMO / LUMO | HOMO = -5.2 eV, LUMO = -3.5 eV [2] |
| Synonyms |
|
| Soluble in | o-xylene, chloroform, chlorobenzene and dichlorobenzene |
| Recommended Processing Solvents | o-xylene (8mg/ml), chloroform (10mg/ml) |
| Classification / Family | Bithiophene, Thienothiophene, Organic semiconducting materials, Low band-gap polymers, Organic photovoltaics, Polymer solar cells, OFETs |
Chemical Structure

Pricing
| Batch | Quantity | Price |
| M0311A | 100 mg | $338 |
| M0311A | 250 mg | $676 |
| M0311A | 500 mg | $1,170 |
| M0311A | 1 g | $2,080 |
| M0311A | 2 g | $3,770 |
| M0311A | 5 g / 10 g* | Please enquire |
*For 5 - 10 grams order quantity, the lead time is 4-6 weeks.
Batch Information
| Batch | Mw | Mn | PDI | Stock info |
|---|---|---|---|---|
| M0311A10 | 130,679 | 46,013 | 2.84 | In Stock |
MSDS Documentation
OFET and Sensing Applications
The exceptional high mobility of this polymer of up to 10 cm2/Vs [2] via solution-processed techniques, combined with its intrinsic air stability (even during annealing) has made PDPP2T-TT-OD of significant interest for OFET and sensing purposes.
While the highest mobilities require exceptional molecular weights of around 500 kD (and with commensurate solubility issues), high mobilities in the region of 1-3 cm2/Vs can still be achieved with good solution-processing at around 250 kD. As such, we have made a range of molecular weights available to allow for different processing techniques.
In our own tests, we have found that by using simple spin-coating onto an OTS-treated silicon substrate (using our prefabricated test chips), high mobilities comparable to the literature can be achieved (1-3 cm2/Vs). Further improvements may also be possible with more advanced strain-inducing deposition techniques.
Photovoltaic Applications
Although shown as a promising hole-mobility polymer for OFETs, when used as the donor material in a bulk heterojunction photovoltaic (with PC70BM as the acceptor), initial efficiencies of 1.6% were achieved for DPP-DTT [3]. The low device metrics were attributed to poor film morphology. However, a higher efficiency of 6.9% was achieved by using thicker film (220 nm) [4].
PDPP2T-TT-OD has also recently been used successfully as an active-layer dopant material in PTB7-based devices [5]. An improvement in device performance was observed, with average efficiencies increasing from 7.6% to 8.3% when the dopant concentration of DPP-DTT was 1 wt%. The use of DPP-DTT as a high-mobility hole-interface layer for perovskite hybrid devices has also been investigated [6].
Synthetic route
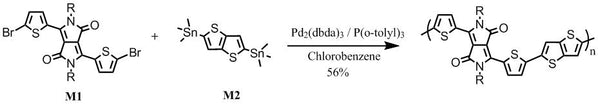
DPP-DTT synthesis: DPP-DTT was synthesised by following the procedures described in [2] and [3] (please refer to the following references):
With 2-thiophenecarbonitrile and dimethyl succinate as starting materials in t-amyl alcohol, it gave 3,6-Dithiophen-2-yl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione. Alkylation of 3,6-Dithiophen-2-yl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione with 2-octyldodecylbromide in dimethylformamide afforded 3,6-bis(thiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione. Further bromination gave 3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (M1).

Further reaction of M1 with 2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene (M2) under Stille coupling conditions gave the target polymer DPP-DTT, which was further purified via Soxhlet extraction with methanol, hexane and then chloroform.
References
- A High Mobility P-Type DPP-Thieno[3,2-b]thiophene Copolymer for Organic Thin-Film Transistors, Y. Li et al., Adv. Mater., 22, 4862-4866 (2010)
- A stable solution-processed polymer semiconductor with record high-mobility for printed transistors, J. Li et al., Nature Scientific Reports, 2, 754, DOI: 10.1038/srep00754 (2012)
- Synthesis of low bandgap polymer based on 3,6-dithien-2-yl-2,5-dialkylpyrrolo[3,4-c]pyrrole-1,4-dione for photovoltaic applications, G. Zhang et al., Sol. Energ. Mat. Sol. C., 95, 1168-1173 (2011)


 DPP-DTT MSDS sheet
DPP-DTT MSDS sheet