Lithium Cobalt Oxide (LiCoO2) Powder
CAS Number 12190-79-3
Battery Materials, Cathode Active Materials, Inorganic Electronic Materials, Materials, Metal OxidesLiCoO2 Powder, Benchmark Battery Materials
High capacity (≥151 mAh/g) LCO powder for lithium-ion battery cathode application
Specifications | Crystal Structure | MSDS | Literature and Reviews | Related Products
Lithium cobalt oxide (LiCoO2 or LCO), CAS number 12190-79-3, is a benchmark battery material that replaces lithium metal as cathode for greater stability and capacity. This high performance LCO cathode material dominates in computer, communication, and consumer electronics-based lithium-ion batteries (LIBs) with the merits of easy procession, unprecedented volumetric and gravimetric energy density, and high operation potential.
LiCoO2 possesses a high theoretical specific capacity (274 mAh/g) and high discharge voltage (~4.2 V vs Li+/Li). However, when half of the Li+ are removed roughly at 4.0 − 4.2 V, it causes an abrupt change in configurational entropy (ΔS) and leads to structural instability. Consequently, commercial LiCoO2 exhibits a maximum capacity of only ~165 mAh/g.
High discharge voltage
(~4.2 V vs Li+/Li)
Worldwide shipping
Quick and reliable shipping
Low Cost
Intrinsic low-cost
Stability & capacity
Greater stability and capacity
Technical Data
| CAS Number | 12190-79-3 |
|---|---|
| Chemical Formula | LiCoO2 |
| Molecular Weight | 97.87 g/mol |
| Chemical Name | Lithium Cobalt Oxide |
| Synonyms | Lithium cobaltite |
| Classification / Family | 2D semiconducting materials, Battery materials, Metal oxides, Cathode materials |
| Colour | Black to grey powder |
Product Detail
| Product Code | M2401A2 |
|---|---|
| Average Particle Size (APS) | D50: 13 μm |
| Specific Surface Area (SSA) | 0.20 m2/g |
| Coin Cell Capacity 1 C (3.0 – 4.3 V) | 154.1 mAh/g |
| True Density | 3.23 g/cm3 |
| Co Content (%) | 59.7 |
| pH | 10.5 |
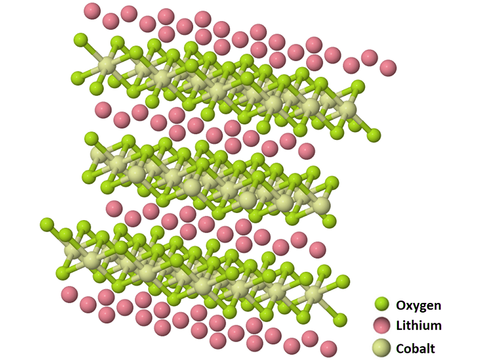
Crystal Structure
The crystal structure of LiCoO2 consists of cobalt atoms that are in the trivalent oxidation state (Co3+), sandwiched between two layers of oxygen atoms. Parallel layers of monovalent lithium cations (Li+) lie between extended anionic sheets of cobalt and oxygen atoms.
*For larger orders please email us to discuss prices.
MSDS Documents
 Lithium Cobalt Oxide (LiCoO2) Powder MSDS Sheet
Lithium Cobalt Oxide (LiCoO2) Powder MSDS Sheet
Literatures
-
Layered lithium cobalt oxide cathodes, A. Manthiram et a., Nat Energy, 6, 323 (2021); DOI: 0.1038/s41560-020-00764-8.
-
Reviving lithium cobalt oxide-based lithium secondary batteries-toward a higher energy density, L. Wang et al., Chem. Soc. Rev., 2018,47, 6505-6602 (2018); DOI: 10.1039/C8CS00322J.
-
Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping, Q. Liu et al., Nat Energy, 3, 936–943 (2018); DOI: 10.1038/s41560-018-0180-6.
Related Products
We stock a wide range of Battery Materials available to purchase online. Please contact us if you cannot find what you are looking for.



