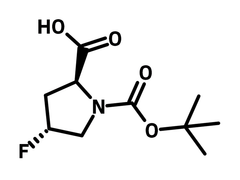
N-Boc-trans-4-fluoro-L-proline
CAS Number 203866-14-2
Chemistry Building Blocks, Fluorinated Building Blocks, Heterocyclic Building Blocks, Materials, MonomersA fluorinated proline with two chiral centres
A synthesis intermediate for APIs, Schiff bases, catalysts and 19F NMR probes
Specifications | MSDS | Literature and Reviews
N-Boc-trans-4-fluoro-l-proline (CAS number 203866-14-2) is a bulky Boc (tert-butoxycarbonyl) group protected l-proline with a fluoride substituent at 4-position. N-Boc-trans-4-fluoro-l-proline is a trans-configured diastereomer, meaning that the fluoride and carboxylic acid are on the opposite sides of the molecule. N-Boc-trans-4-fluoro-l-proline reacts with 2-fluoro-5-nitrobenzaldehyde (nucleophilic aromatic substitution) producing a 19F NMR chiral probe to determine the enantiomeric purity of chiral amino acids. N‑Arylsulfonyl‑l‑proline derivatives synthesized from N-Boc-trans-4-fluoro-l-proline is applied as a potent and selective αvβ1 integrin inhibitors. It shows inhibitive performance at half maximal inhibitory concentration (IC50) of 0.02 nM.
The carboxylic acid and the secondary amine of N-Boc-trans-4-fluoro-l-proline can coordinate to metal centres, forming a stable 5-membered ring. A Pd (II) complex with l-proline ligands has been explored yielding enantiomeric excess up to 24% in catalytic olefination reaction.
Multiple functional groups
For facile synthesis
Fluorinated L-proline building block
For drug discovery, NMR probe and organic synthesis
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
General Information
| CAS Number | 203866-14-2 |
| Chemical Formula | C10H16FNO4 |
| Full Name | (2S,4R)-1-tert-Butoxycarbonyl-4-fluoro-2-pyrrolidinecarboxylic acid |
| Molecular Weight | 233.24 g/mol |
| Synonyms | N-(tert-Butoxycarbonyl)-trans-4-fluoro-l-proline, (4R)-1-(tert-Butoxycarbonyl)-4-fluoro-l-proline, Boc-4F-l-Proline |
| Classification / Family | Fluorinated building blocks, Heterocyclic building blocks, Chiral building blocks, APIs, Schiff bases, Catalysts, 19F NMR probe |
Chemical Structure
Product Details
| Purity | 97% |
| Melting Point | Tm = 115 – 119 °C |
| Optical Rotation | -70.69 ° (c = 1, methanol) |
| Appearance | White to off-white powder |
MSDS Documentation
 N-Boc-trans-4-fluoro-l-proline MSDS Sheet
N-Boc-trans-4-fluoro-l-proline MSDS Sheet
Literature and Reviews
-
Exploring N-arylsulfonyl-L-proline scaffold as a platform for potent and selective αvβ1 integrin inhibitors, N. Reed et al., ACS Med. Chem. Lett., 7, 902−907(2016); DOI: 10.1021/acsmedchemlett.6b00196.
-
Synthesis, Ni(II) Schiff base complexation and structural analysis of fluorinated analogs of the ligand (S)-2-[N-(N'-benzylprolyl)amino]benzophenone (BPB), N. Tatum et al., J. Fluor. Chem., 173, 77−83(2015); DOI: 10.1016/j.jfluchem.2015.02.007.
-
Synthesis, structure, and catalytic reactivity of Pd(II) complexes of proline and proline homologes, D. Hobart et al., Catalysts, 9, 515(2015); DOI: 10.3390/catal9060515.
