NDI-2HD-2Br
CAS Number 1459168-68-3
Chemistry Building Blocks, Dibromo Monomers, Heterocyclic Building Blocks, Materials, MonomersSpecifications | MSDS | Literature and Reviews
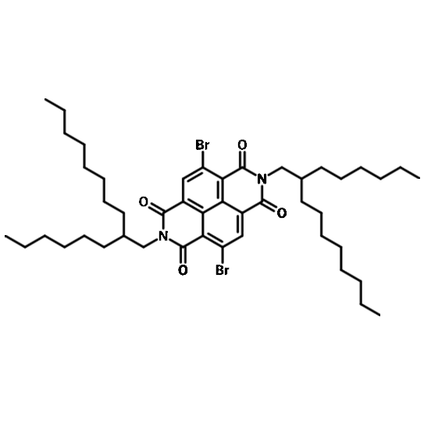
With large branched side-chains, 4,9-Dibromo-2,7-bis(2-hexyldecyl)benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetraone (also known as NDI-2HD-2Br) adds solubility to polymer semiconductors by functioning as repeating units in the back-bone.
Polymers prepared from this monomer, such as PNDI(2HD)T, PNDI(2HD)2T and PNDI(2HD)S, are p-type high-mobility polymers used for high-performance OFETs and all-PSCs.
NDI building block
For the synthesis of OFET, OPV, and OLED materials
Worldwide shipping
Quick and reliable shipping
Functionalized with bromides
for facile reactions
High purity
>98% Purity
General Information
| CAS Number | 1459168-68-3 |
| Chemical Formula | C46H68Br2N2O4 |
| Molecular Weight | 872.85 g/mol |
| Synonyms |
4,9-Dibromo-2,7-bis(2-hexyldecyl)benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetraone NDI2HD-Br2 NDI(2HD)2Br |
| Classification / Family | Benzophenanthroline, Organic semiconducting materials, Semiconductor Synthesis, Low band gap polymers, OFETs, OLED, Organic photovoltaics, Polymer solar cells |
Chemical Structure
Product Details
| Purity | >98% |
| Melting Point | N/A |
| Appearance | Light yellow powder/crystals |
MSDS Documentation
Literature and Reviews
- Improved All-Polymer Solar Cell Performance by Using Matched Polymer Acceptor, S. Shi et al., Adv. Funct. Mater., 26, 5669–5678 (2016); DOI: 10.1002/adfm.201601037.
- Flexible, highly efficient all-polymer solar cells, T. Kim et al., Nat. Commun., 6, 8547 (2015); DOI: 10.1038/ncomms9547.
- Impact of highly crystalline, isoindigo-based small molecular additives for enhancing the performance of all-polymer solar cells, H-H. Cho et al., J. Mater. Chem. A, 5, 21291–21299 (2017); DOI: 10.1039/c7ta06939a.
-
Comparative Study of Thermal Stability, Morphology, and Performance of All-Polymer, Fullerene–Polymer, and Ternary Blend Solar Cells Based on the Same Polymer Donor, T. Kim et al., Macromolecules, 50 (17), 6861–6871 (2017);
DOI: 10.1021/acs.macromol.7b00834.

 NDI-2HD-2Br MSDS Sheet
NDI-2HD-2Br MSDS Sheet