PBBTCD, high quality semiconducting polymer
High purity and available online for priority dispatch
Specifications | MSDS | Literature and Reviews
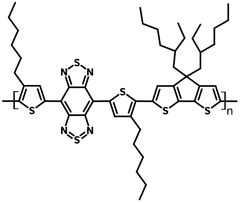
PBBTCD, polybenzobisthiadiazole-dithienocyclopentane (CAS number 1334032-14-2), is a small bandgap copolymer (Eg = ~ 0.6 eV) with a backbone alternating electron donating cyclopentadithiophene (CDT) and electron accepting fused-ring benzobisthiadiazole (BBT) units.
High charge mobilities were observed to be 0.076 cm2 V-1 s-1 for electrons and 0.10 cm2 V-1 s-1 for holes for PBBTCD due to its planar structure and orderly packed structure in film. The balanced ambipolarity of the BBT moiety of the polymer makes it a good candidate for logic circuit applications.
For 5 - 10 grams order quantity, the lead time is 4-6 weeks.
Luminosyn™ PBBTCD
Luminosyn™ PBBTCD is now available.
High purity
PBBTCD is purified via Soxhlet extraction with acetone, hexane, and chlorobenzene under an argon atmosphere
Large quantity orders
Plan your experiments with polymers from the same batch
General Information
| Full name | Poly[(4,7-bis(3-hexylthien-2-yl)- 2λ4δ2-benzo[1,2-c;4,5-c′]bis[1,2,5] thiadiazole)-alt-(3,3-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b′]dithiophene)] |
| Synonyms | PBBTCD |
| Chemical formula | (C51H64N4S6)n |
| CAS number | 1334032-14-2 |
| HOMO / LUMO | HOMO = -4.80 eV, LUMO = -4.00 eV; Eg = 0.6 eV [1] |
| Soluble in | Chloroform, chlorobenzene and dichlorobenzene |
| Recommended Processing Solvents at 10mg/ml | Chlorobenzene |
| Classification / Family | Organic semiconducting materials, Very low-bandgap polymers, Ambipolar semiconducting polymers, OFET polymers, High charge mobility polymers, Photodetectors, Thin-film Transistors |
Batch details
| Batch | Mw | Mn | PDI | Stock Info |
| M2256A1 | 17,762 | 7,733 | 2.30 | In stock |
Chemical Structure
UV-Vis-NIR Absorption

MSDS Documentation
Literature and Reviews
- Ambipolarity in Benzobisthiadiazole-Based Donor–Acceptor Conjugated Polymers, J. D. Yuen et al., Adv. Mater., 23, 3780–3785 (2011); DOI: 10.1002/adma.201101134.
- Infrared spectroscopy of narrow gap donor-acceptor polymer-based ambipolar transistors, O. Khatib et al., Phys. Rev. B 86, 195109 (2012); DOI: 10.1103/PhysRevB.86.195109.
- Organic Transistors in the New Decade: Toward n-Channel, Printed, and Stabilized Devices, S. Kola et al., J. Polym. Sci. B: Polym. Phys., 50, 1090–1120 (2012); DOI: 10.1002/polb.23054.

 PBBTCD MSDS sheet
PBBTCD MSDS sheet