PDBPyBT (DPPDPyBT, high-mobility n-type polymer)
CAS Number 1455028-36-0
Chemistry Building Blocks, Interface Polymers, Materials, OFET & OLED Polymer Materials, OPV Polymers, Semiconducting PolymersPDBPyBT, electron transporting material for OFETs and OPVs
High purity and available online for priority dispatch
Specifications | Pricing and Options | MSDS | Literature and Reviews
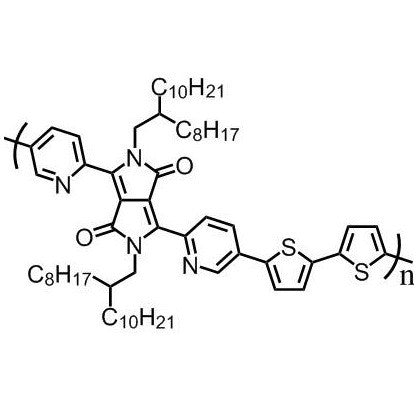
Poly(2,5-bis(2-octyldodecyl)-3,6-di(pyridin-2-yl)-pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione-alt-2,2’-bithiophene) (DPPDPyBT), CAS number 1455028-36-0, is a good electron transporting material used in the application of OFETs and OPVs.
PDBPyBT (DPPDPyBT, high-mobility n-type polymer) from Ossila was used in the high-impact paper (IF 10.51), Versatile Interpenetrating Polymer Network Approach to Robust Stretchable Electronic Devices, G. Zhang et al., Chem. Mater., 29, 18, 7645–7652 (2017); DOI: 10.1021/acs.chemmater.7b03019. Synergistic Use of Bithiazole and Pyridinyl Substitution for Effective Electron Transport Polymer Materials, C. Buckley et al., Chem. Mater., 31, 11, 3957–3966 (2019); DOI: 10.1021/acs.chemmater.9b00208.
DPPDPyBT contains a DPP unit which has been found to be an excellent electron accepting moiety for a large number of polymer semiconductors for OTFTs, OPVs and OLEDs. DPPDPyBT has been proven to have a planar structure, together with two five-membered rings having six π-electrons being shared, an electron rich moiety with DPP and two pyridyl units being excellent electron withdrawing ability, resulting in DPPDPyBT being an excellent candidate as an electron transporting material.
As one of promising high charge carrier mobility polymers (electron and hole mobility up to 6.3 cm2V-1s-1 and 2.78 cm2V-1s-1 respectively, has been reported [1]), these type of polymers are also used as blending material (low ratio) for OPV devices to facilitate electron collection from the active layer to PCBMs [2].
PDBPyBT, a high-mobility n-type polymer, is now back in stock with higher molecular weight.
General Information
| CAS number | 1455028-36-0 |
| Chemical formula | (C64H92N4O2S2)n |
| HOMO / LUMO | HOMO = 5.69 eV, LUMO = 4.33 eV [1] |
| Synonyms |
|
| Soluble in | Chloroform, chlorobenzene, dichlorobenzene |
| Recommended Processing Solvents at 10mg/ml | Chlorobenzene (2mg/ml) at elevated temperature ca. 100 °C |
| Classification / Family | Pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione, Bithiophene, Heterocyclic five-membered ring, Organic semiconducting materials, Low band gap polymers, Organic Photovoltaics, Polymer Solar Cells, OFETs, All-polymer solar cells. |
Batch Details
| Batch number | MW | Mn | PDI | Stock info |
|---|---|---|---|---|
| M0321A1 | 472,567 | 90,532 | 5.22 | In stock |
Chemical Structure
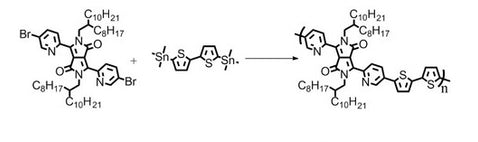
Synthetic Route
PDBPyBT was synthesised engaging Stille Coupling reaction with 3,6-Bis(5-bromopyridin-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione and 5,5′-bis(trimethylstannyl)-2,2-bithiophene as starting materials in chlorobenzene as solvent. Polymer was purified by Soxhlet extraction with methanol, acetone, hexane and chlorobenzene as washing and extracting solvents.
Pricing
| Batch | Quantity | Price |
| M0321A |
50 mg | £330 |
| M0321A | 100 mg | £600 |
| M0321A | 250 mg | £1400 |
| M0321A | 500 mg | £2500 |
| M0321A | 1 g / 5 g* | Please enquire |
*for 1 - 5 grams order quantity, the lead time is 4-6 weeks.
MSDS Documentation
Literature and Reviews
- Record High Electron Mobility of 6.3 cm2V-1s-1 Achieved for Polymer Semiconductors Using a New Building Block, B. Sun et al., Adv. Mater., 26, 2636-2642 (2014)
- Enhanced efficiency of polymer solar cells by adding a high-mobility conjugated polymer, S. Liu et al., Energy Environ. Sci., 8, 1463-1470 (2015)
- A pyridine-flanked diketopyrrolopyrrole (DPP)-based donor–acceptor polymer showing high mobility in ambipolar and n-channel organic thin film transistors, B. Sun, Polym. Chem., 6, 938-945 (2015)

 PDBPyBT MSDS sheet
PDBPyBT MSDS sheet