PFN, PFN-DOF
CAS Number 673474-74-3
Cathode Interlayer Materials (CIMs), Interface Polymers, Materials, Organic Conductors, Perovskite Interface Materials, Perovskite Materials,PFN, high quality semiconducting polymer
Available for fast, secure dispatch
Overview | Specifications | MSDS | Pricing and Options | Literature and Reviews
PFN (CAS number 673474-74-3) is a conjugated polyelectrolyte used as an electron-interface in OPV devices to improve extraction efficiencies. Currently producing power conversion efficiencies of up to 7.1% at Ossila, with further increases expected from additional optimisation and up to 9.2% reported in the literature [1-3].
Cathode interlayer molecule
For high efficient OPV applications
Improve extraction efficiency
as a conjugated polyelectrolyte
Worldwide shipping
Quick and reliable shipping
Good solubility
Soluble in polar solvents with small amout of acetic acid
PFN from Ossila was used in the high-impact paper (IF 30.85), All-Organic and Fully-Printed Semitransparent Photodetectors Based on Narrow Bandgap Conjugated Molecules, G. Pace et al., Adv. Mater., 26, 6773-6777 (2014); DOI: 10.1002/adma.201402918.
Soluble in polar solvents such as water and methanol in the presence of small amounts of acetic acid.
General Information
| Full name | Poly [(9,9-bis(3'-(N,N-dimethylamino)propyl)-2,7-fluorene)-alt-2,7-(9,9–dioctylfluorene)] |
| Synonyms |
|
| Chemical formula | (C52H70N2)n |
| CAS number | 673474-74-3 |
Chemical Structure
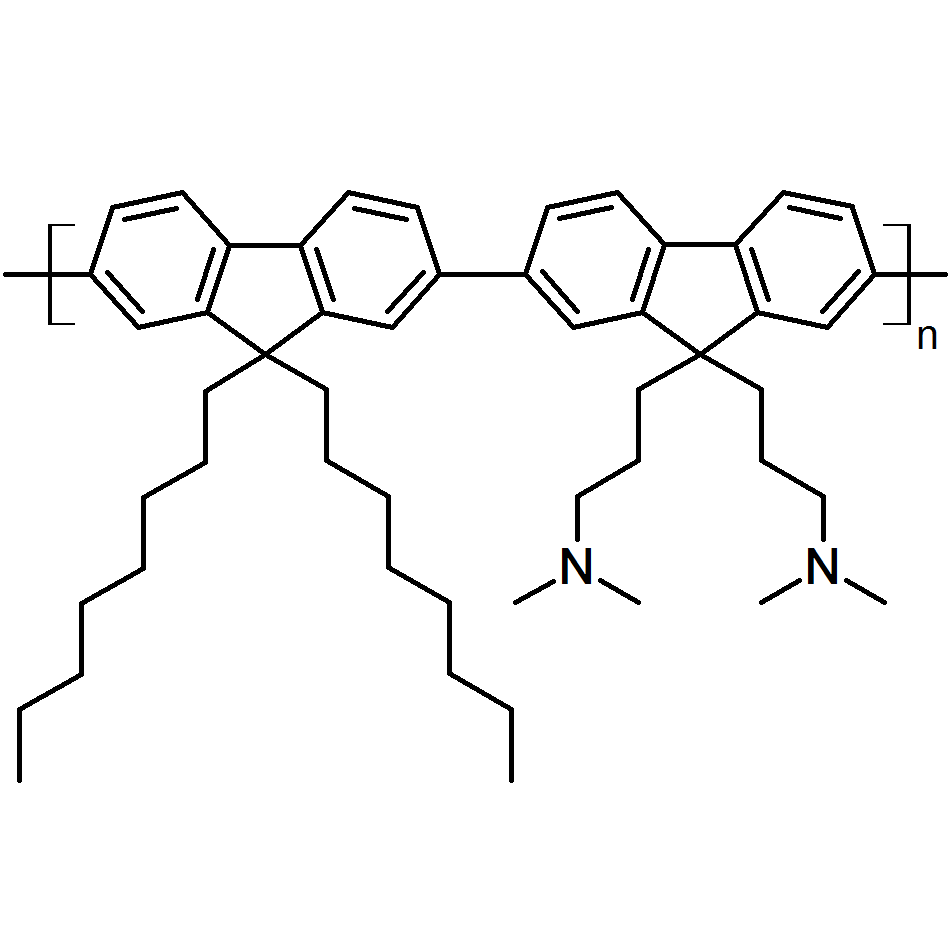
Usage details
Inverted OPV devices were made using the architecture shown below with PFN as an electron-interface and PTB7:PC70BM in a 1:1.5 blend ratio. Ossila's pixelated cathode substrate pack (S213) provided the device components.
Glass / ITO (100 nm) / PFN (5.5 to 10 nm) / PTB7:PC70BM (90 nm) / MoOx (15 nm) / Al (100 nm)
The substrate cleaning and PFN spin-coating were performed under ambient conditions with all other steps performed in an N2 glove box until encapsulation had been completed (measurement performed under ambient conditions).
The active layer thickness, MoOx thickness, cathode metal (Ag or Al), PFN solution concentration, PFN drying/baking have not been fully optimised. As such, we expect further gains to be made with additional engineering work. However, for the devices made in this fabrication, a peak efficiency of 7.1% was achieved.
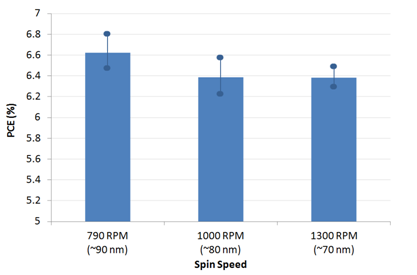
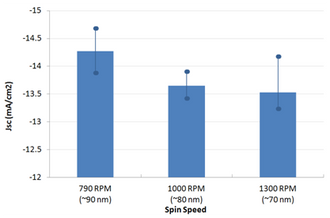
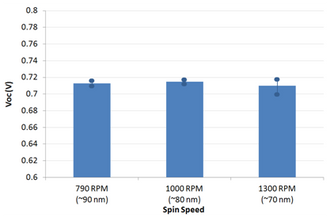
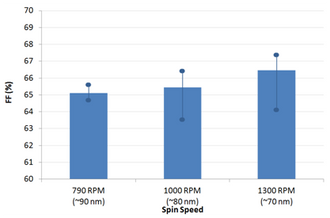
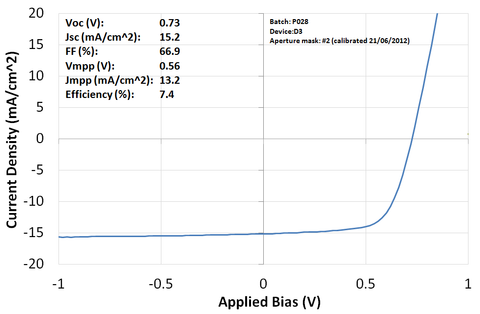
Note that some burn-in was observed (i.e. a small improvement in device performance after a few seconds under the solar simulator) and the variability of the devices is currently slightly higher than for other interlayers (average PCE of 6.7%). We expect the uniformity to improve with further improvements in PFN processing, in particular the optimisation of drying conditions to ensure that the acetic acid is fully removed prior to active layer deposition.
Fabrication Routine
The below fabrication routine was used to fabricate inverted solar cells with peak efficiency of 7.1%. Further gains are expected with further optimisation.
Cleaning Substrates
- Pixelated Cathode substrates (S213)
- 5 mins sonication in hot Hellmanex III (1 ml in beaker)
- 2x boiling water dump rinses
- 5 mins sonication in warm IPA
- 2x dump rinses
- 5 mins sonication in hot NaOH
- Dump rinse in boiling water
- Dump rinse in water
- Stored in DI water overnight and until use
PFN solution
- Stock solution of acetic acid dissolved 1:9 in methanol to enable low concentration solutions to be made more easily.
- Acetic acid solution further dissolved to produce 2 μl/ml solution.
- PFN dissolved at 2 mg/ml in methanol with 2 μl/ml of acetic acid with stir bar at ambient temperature for 10 minutes
- Filtered through 0.45 µm PES filter
PFN Test Films
- PFN Test film initially spun at 500 rpm and gave 21-22 nm
- Second test film spun at 1000 rpm and gave 13-16 nm
- Thicknesses extrapolated for higher spin speeds
- It was noted that at low spin speeds 500 rpm to 2400 rpm there were significant crystallites present in the films - especially on the ITO. Extra filtration showed that this was not due to the solution and therefore most have been due to the drying process
Active Layer Solution
- Fresh stock solution of PTB7 made on at 10 mg/ml in CB and dissolved with stir bar for 1 hour (dissolves very easily)
- Mixed 1:1.5 with dry Ossila 95/5% C70 PCBM to make overall concentration of 25 mg/ml and dissolved with stir bar for 1 hour more
- 3% of diiodooctane (DIO) added to solution
- Filtered using 0.45 μm PTFE (hydrophobic) syringe filter
Active Layer Test Films
- Test film spun at 1000 rpm for 2 mins using unfiltered solution and thickness measure on Dektak. Note that films must be fully dry before performing Dektak measurements.
- 1000 rpm gave approximately 90 nm thickness.
Active layers
- Devices spun using 30 μl dynamic dispense (20 μl gave only moderate wetting/coverage)
- Spun for 2 mins
- Cathode wiped with CB
- Vacuum dried in glove box antechamber for 20 mins to remove residual DIO from films
Cathode Evaporation
- 15 nm of MoOx evaporated at 0.2 Å/s from fresh pellets at pressure 1e-6 mbar
- 100 nm of Al evaporated at 1.5 Å/s at pressure 1e-6 mbar
Annealing/Encapsulation
- No annealing performed
- Encapsulated as standard, using Ossila encapsulation epoxy (E132) and glass coverslips (C181) (30 mins in UV light box).
Measurements
- JV sweeps taken with Keithley 237 source-meter
- Illumination by Newport Oriel 9225-1000 solar simulator with 100 mW/cm2 AM1.5 output
- NREL certified silicon reference cell used to calibrate lamp output
- Lamp current: 7.9 A
- Solar output at start of testing: 0.995 suns at 25°C
- Solar output at end of testing: 1.00 suns at 25°C
- Electrochemically etched aperture mask was optically calibrated to 0.212 cm2
MSDS Documentation
Pricing
| Batch | Quantity | Price |
| M222 | 100 mg | £280 |
| M222 | 250 mg | £560 |
| M222 | 500 mg | £900 |
| M222 | 1 g | £1400 |
Literature and References
- Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure, Z. He et al., Nature Photonics, 6, 591–595 (2012)
- Simultaneous Enhancement of Open-Circuit Voltage, Short-Circuit Current Density, and Fill Factor in Polymer Solar Cells, Z. He et al., Advanced Materials, 23, 4636–4643 (2011)
- Investigation of a Conjugated Polyelectrolyte Interlayer for Inverted Polymer:Fullerene Solar Cells, R. Xia et al., Advanced Energy Materials, (2013)

 PFN MSDS sheet
PFN MSDS sheet