1,3,6,8-Tetrakis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene
CAS Number 1398053-00-3
Boronates, Chemistry Building Blocks, COF Ligands, Materials, Monomers, Porous Organic FrameworksCovalent organic frameworks (COFs) Pyrene ligand
Tetratopic bridging ligand for the synthesis of COFs in application of neutron detection, scintillator and hydrogen evolution reactions (HERs)
Specifications | MSDS | Literature and Reviews
1,3,6,8-tetrakis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene is a pyrene with four boronic acid pinacolate ester side groups at 1,3,6,8-positions. Pyrene tetraboronic ester is mechanically ground fluorescence active but phosphorescence inactive. It exhibits room temperature phosphorescence in air with mechanical force due to the good communication between singlet and triplet states from the efficient intermolecular electronic coupling in the dimer formed upon scratching.
Being boron rich, 1,3,6,8-tetrakis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene finds application in n neutron detection. By blending the fluorescent dopant in poly(vinyltoluene) matrice, it shows comparable scintillation light output and neutron capture as state-of-the art commercial scintillator.
Conjugated microporous polymer PyDF with alternating acceptor unit of fluorinated dibenzothiophene-dioxide (DBTDO) and donor units of pyrene shows excellent photocatalytic activity with an attractive photocatalytic hydrogen generation rate of 18.93 mmol h−1 g−1, thanks to the efficient separation of light-generated electrons and holes.
MOF and COF ligands
Boronic ester ligand for cross-linked COF networks
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
Facile reactions
Readily polycondensation cross-coupling reactions to form COF networks
General Information
| CAS Number | 1398053-00-3 |
| Chemical Formula | C40H54B4O8 |
| Full Name | 1,3,6,8-Tetrakis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene |
| Molecular Weight | 706.1 g/mol |
| Synonyms | TPPY, 1,3,6,8-Tetra(pinacolboryl)pyrene |
| Classification / Family | COFs, Pyrenes, Boronic esters, HERs |
Chemical Structure
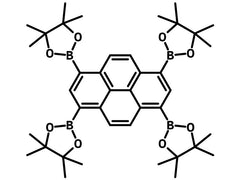
Product Details
| Purity | > 97% |
| Melting Point | N/A |
| Appearance | Beige to yellow powder/crystals |
MSDS Documentation
 1,3,6,8-Tetrakis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene MSDS Sheet
1,3,6,8-Tetrakis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene MSDS Sheet
Literature and Reviews
-
Mechano-responsive room temperature luminescence variations of boron conjugated pyrene in air, V. Wakchaure et al., Chem. Commun., 54, 6028-6031 (2018); DOI: 10.1039/C8CC03494J.
-
Substituent effect of conjugated microporous polymers on the photocatalytic hydrogen evolution activity, X. Gao et al., J. Mater. Chem. A, 8, 2404-2411 (2020); DOI: 10.1039/C9TA13212K.
-
Boron-rich benzene and pyrene derivatives for the detection of thermal neutrons, H. Yemam et al., Sci. Rep., 5, 13401 (2015); DOI: 10.1038/srep13401.
