Anode vs Cathode: What is the Difference?

Anodes and cathodes are important components of how a battery works. The difference between, and the roles of, a cathode and an anode are easily confused. They are often described as the positive and negative electrodes. Yet, this definition does not sufficiently explain cathodes and anodes in all systems.
Defining Current
Defining a cathode and anode as positive and negative, or as the source and sink of a current, depends on your definition of current itself. Current can describe the flow of positive or negative charge:
- Conventional current describes the flow of positive charge. This is despite the fact electrons constitute the actual electrical flow.
- Electron current describes the actual flow of negative electrons.
The flow direction of electrons and positive ions depends on the state of the battery. Thus, it is important to describe the system further when defining the difference between an anode and a cathode.
Anodes and Cathodes
What is a Cathode?
For intercalation-based batteries, such as lithium-ion batteries, the cathode supplies the positive ions that allow for intercalation with the anode.
The battery materials used influence the intercalation process. Lithium-ion batteries use lithium ions (Li+), while sodium-ion batteries use sodium ions (Na+). The chemistry and structure of the cathode is selected to enhance the discharge rates and overall capacity of the battery.
The intercalation process is dependent on the cathode and is the foundation for how batteries work. As the source of positive ions, cathodes are typically the most complicated and important element of a battery.
While research into all the components of a battery can push the experimental capacity closer to the theoretical, the cathode requires the most development. The cathode material can be designed to tackle different tasks with certain chemistries offering better cycle life in exchange for power output, and vice versa.
Examples of cathode active materials
What is an Anode?
In intercalation-based batteries, the anode is the intercalation point for positive ions.
Typically, the anode requires a porous structure so intercalation can occur, and it must be electronically conductive. Other material qualities considered include thermal and electrical isometry. An anode that expands or contracts during intercalation or heating can cause battery failure.
Carbon nanotubes and graphene based materials make excellent anode active materials due to their high electron conductivity, stability and low weights.
Examples of anode active materials
The Role in a Battery
Charging
During charging, the cathode supplies ions and electrons. The anode stores the positive ions through intercalation and hold the electrons ready for discharge.
In a lithium-ion battery, when you apply an electric voltage, the lithium ions are 'pushed' through the electrolyte from the cathode to the anode. These ions will then intercalate with the anode, holding the positive charge until discharging occurs.
During this process, electrons also move from the cathode to the anode through the circuit. Once the charging circuit is disconnected, the electrons are held at the anode ready for discharge. The non-conductive nature of the electrolyte prevents the electrons from returning to the cathode.
In a rechargeable battery, this process occurs each time the battery is charged.
Discharging
As the battery is used, or discharged, the anode releases electrons and ions. Connecting the battery to a circuit allows this discharge. This provides a path for the flow of electrons, which defines electrical current, through the circuit. The release of electrons triggers the ions intercalated with the anode to flow back to the cathode through the electrolyte.
Once the battery is fully discharged, the cathode holds the electrons and ions. This process is also repeated in rechargeable batteries.
Summary
Cathodes and anodes are key parts of a battery. While often labelled as either positive or negative, these terms do not fully describe their roles. The direction of current, either as positive charge flow or actual electron flow, also matters. Whether the battery is charging or discharging determines which electrode supplies the flow of electrons. In both processes, a flow of negative electrons and positive ions occurs at the same time, in the same direction. Therefore, the positive and negative terminals are not the same in all cases.
Related Products
Learn More
 An Introduction to Batteries
An Introduction to Batteries
Typically, batteries work by a process known as intercalation. This process occurs across the battery components. Most batteries consist of the same components.
Read more...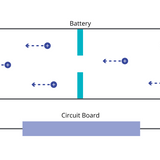 How Lithium-Ion Batteries Work
How Lithium-Ion Batteries Work
Lithium-ion batteries use the reversible lithium intercalation reaction. The battery has several important components to enable this intercalation.
Read more...











