Battery Materials: The Key to High-Performance Energy Storage

Battery materials are the components that make up a battery, each serving a specific role in storing and harnessing electrical energy. The most well-known components are the electrodes (cathode and anode). The materials used for these critical components, known as cathode and anode active materials, play a key role in supplying and intercalating charged species necessary for electricity generation. Whether it’s a lithium-ion, sodium-ion, nickel, solid-state or other battery technology, the key components of a battery remain mainly unchanged.
Cathode Active Materials
Cathode active materials are a key component of any battery. Many of these materials are high purity metal oxides that are capable of releasing charged species during the charging process, that go on to reach and be stored in the anode. Cathode active materials are able to undergo electrochemical redox reactions safely, giving the battery rechargeable qualities. This involves metals changing oxidation state during charge and discharge cycles.
These battery materials possess excellent conductive properties, efficiently transporting charge between current collectors. The choice of cathode material significantly impacts a battery’s overall energy density, which is determined by cell voltage and capacity—essentially, how many releasable charged species the material can provide.
In lithium-ion battery technology, the cathode active material supplies lithium ions, existing in a delithiated state when the battery is fully charged. It plays a crucial role in energy storage by directly influencing the number of lithium ions that can be stored and released. Consequently, cathode active materials are required in large quantities for battery production. There are six main types of lithium-ion cathode active material: LFP, LMO, LCO, NMC, NCA, NCM, as well as the newer LNMO.
Anode Active Materials
Anode active materials are essential for efficient battery operation, serving as the site of reduction and the source of electrons in intercalation-type batteries like lithium-ion. These materials must be capable of hosting and releasing charged species during charge and discharge cycles while maintaining structural integrity and stability.
The most common anode active materials are carbon-based, particularly graphite, which is widely used due to its excellent conductivity, stability, and ability to intercalate lithium ions efficiently. Carbon black is also utilized in some cases to enhance conductivity.
Beyond traditional carbon anodes, lithium titanate (LTO) is emerging as an alternative anode material. Unlike graphite, LTO has a higher intercalation potential, reducing the risk of lithium plating and improving safety. It also offers exceptional cycle stability, fast charging capabilities, and a long lifespan, making it ideal for applications requiring high durability, such as electric buses and grid storage. The choice of anode material significantly impacts battery performance, affecting energy density, cycle life, and overall efficiency.
Explore the range of high-purity cathode materials designed for high-capacity, high-voltage batteries to maximize energy density.
Current Collector
Current collectors are the battery materials that the anode and cathode active materials are coated on. These materials are directly connected to the external circuit. They are typically a thin layer of metal that carries electrons to and from the active materials. They directly impact the battery’s capacity, stability and performance. Common current collectors are aluminum and copper foil. Active electrode slurries can be coated on their surfaces easily using a range of coating techniques that can be scaled up for mass production.

What are Battery Slurries?

Battery slurries are liquid mixtures used in the production of battery electrodes. They consist of active materials, conductive additives, binders, and solvents, which are combined to form a homogenous paste. This slurry is then coated onto a metal foil (typically aluminum for the cathode and copper for the anode), dried, and processed into electrode sheets.
Slurries ensure even distribution of active materials, optimizing electrochemical performance. They affect the electrode quality which directly impacts battery capacity, lifespan and safety.
Different coating techniques are used to coat battery slurries onto the current collector foils. These methods include, slot-die coating, doctor blade coating and dip coating.
Battery Electrolyte
Battery electrolytes facilitate the movement of charged species between the two electrodes, enabling the generation and storage of electrical energy. It can be in liquid, solid, or gel form and typically consists of a solvent and a dissolved salt (such as LiPF₆ for lithium-ion batteries) to provide mobile ions. The electrolyte's composition affects the battery's performance, safety, and lifespan by influencing conductivity, thermal stability, and electrochemical reactions.
Electrolyte additives can be used to solves issues of electrolyte degradation, unwanted growths on the anode and cathode dissolution. They can be referred to as redox shuttle molecules and become involved in the electrochemical redox reactions to increase the efficiency of the battery.
Battery Separator
The separator material provides structural integrity and serves as a safety feature by moderating ion transport between the electrodes. If ions move too quickly through the electrolyte, it can increase the risk of thermal runaway. To mitigate this, separators are designed with thermal shutdown mechanisms, where their pores close at high temperatures to help prevent dangerous reactions involving other battery materials.
An important area of research focuses on separator design to prevent dendrite formation and solid electrolyte interphase (SEI) degradation, both of which impact battery lifespan. Uneven lithium-ion movement through the separator can lead to dendritic growth, where needle-like lithium structures penetrate the separator, increasing the risk of short circuits. By maintaining uniform ion distribution, separators can help suppress dendrite growth and improve battery longevity and safety.
Inorganic Electronic Materials

Battery Polymers
While the primary function of a battery is to store and release energy through metal-based or metal-ion-based reactions, polymers play an equally crucial role in ensuring battery performance, stability, and longevity. Nearly every modern battery would fail to operate efficiently without polymers, as they contribute to multiple essential functions within the battery cell, including:
- Electrode Binders - Polymers help hold active materials together in electrode slurries, ensuring mechanical stability during charging and discharging. Common binders include PVdF (for cathodes) and SBR (for anodes).
- Separators and Membranes - Battery separators, made from polyethylene (PE) or polypropylene (PP), prevent short circuits while allowing ion flow between electrodes.
- Active Materials - Some advanced batteries use polymers as charge-storing materials, such as conducting polymers or organic redox-active compounds.
Polymer based battery materials improve stability, enable efficient ion transport, and contribute to battery safety. Their role is vital in both conventional and emerging battery technologies.
Conductive Additives
Conductive additives are used to enhance an electrode’s charge and discharge performance. They are used to reduce the contact resistance of an electrode to accelerate device efficiency. They improve the transport rate of charged species in the electrode material. As a result, the lifespan of the whole battery is improved.
Common conductive additives include:
- Carbon nanotubes - Offer high electrical conductivity, mechanical strength, and a large surface area. They form a conductive network in electrodes, reducing resistance and improving charge transfer while preventing mechanical degradation. CNTs are used in lithium-ion batteries, supercapacitors, and solid-state batteries.
- Graphene Materials - Lightweight, highly conductive, and mechanically strong. Its two-dimensional structure enhances ion diffusion and electron transport, improving battery performance. It is commonly found in high-performance lithium-ion and sodium-ion batteries.
- Barium titanate - A ferroelectric material with a high dielectric constant. It enhances charge storage, conductivity, thermal stability, and mechanical strength, making it valuable for solid-state batteries and advanced energy storage.
Thermal Management Materials
Thermal management materials regulate temperature in batteries, preventing overheating and improving performance, safety, and lifespan. These battery materials help dissipate excess heat, insulate components, and enhance thermal stability.
- Phase change materials (PCMs) absorb and release heat during phase transitions, maintaining stable battery temperatures.
- Thermal interface materials (TIMs), such as thermal pastes and gels, improve heat transfer between components.
- Heat spreaders, like graphene sheets, carbon nanotubes, distribute heat evenly.
- Ceramic coatings provide thermal insulation and fire resistance.
- Liquid cooling systems use coolants to actively remove heat in high-power applications.
Learn More
 An Introduction to Batteries
An Introduction to Batteries
Typically, batteries work by a process known as intercalation. This process occurs across the battery components. Most batteries consist of the same components.
Read more...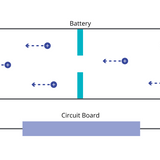 Lithium-Ion Battery Components and Working Principle
Lithium-Ion Battery Components and Working Principle
Lithium-ion batteries operate based on electrochemical reactions, specifically redox reactions involving lithium and sometimes other redox-active elements. These reactions result in the movement of lithium ions between the electrodes and the flow of electrons through an external circuit.
Read more...Contributors
Written by
Application Scientist
Diagrams by
Graphic Designer


