What is a Photosensitizer?
A photosensitizer is a molecule that absorbs light and converts it to a different form of energy. This energy is typically transferred to other molecules through an energy transfer mechanism, such as Förster or Dexter transfer. Photosensitive materials are useful for a range of applications from photodynamic medical treatments to harnessing solar energy.
A photosensitizer is typically sensitive to specific wavelengths of light. If a broader optical range is needed, a combination of photosensitizers can be used. A well-known naturally occurring photosensitizer is chlorophyll, which converts sunlight into energy for plant growth by initiating photosynthesis.
Photosensitizer Theory
Photosensitizers operate through energy transfer mechanisms, enabling the transfer of absorbed light energy to other molecules or systems. By analyzing the kinetics of energy transfer within photosensitizer systems, researchers can gain valuable insights into the materials' behavior. This analysis helps determine key properties such as efficiency, photostability, and reaction dynamics.
Photosensitization Mechanism
Upon excitation by light (a photon), a photosensitizer is able to transfer energy to an acceptor or quencher molecules. Typically, this happens via a triplet-triplet energy transfer. This describes the mechanism of an excited triplet state electron from the photosensitizer (donor) to the triplet state of the acceptor/quencher molecule.
Photosensitive molecules are usually excited by the absorption of photons of a specific wavelength. This initiates electron excitation into the singlet excited state. From here the electron can move to a triplet excited state via intersystem crossing (KISC). If the triplet state of the donor molecule is higher than that of the acceptor molecule the electron can transfer between the states. Thereby populating the triplet state of the acceptor via energy transfer (KET). This process, known as Dexter energy transfer, is a short-range interaction that requires orbital overlap between the donor and acceptor molecules.
Compounds that can be efficiently excited to the triplet excited state are known as triplet photosensitizers. They can then act as catalysts in photochemical reactions which make them useful for a variety of applications including photodynamic therapy.
Kinetics of Photosensitization
The kinetics of photosensitization is dictated by the transfer of energy between excited triplet states. The photosensitizer donor will interact with the acceptor molecule if it is within the required distance.
The quantum yield for net energy transfer (φet) between donor and acceptor molecule is given by the following equation:
|
φisc = quantum yield of intersystem crossing [A0] = acceptor concentration |
ket = rate constant for net triplet energy transfer kd = donor triplet decay |
In order to solve the equation, the quantum yield of intersystem crossing with the fluorescence lifetime (𝜏f) is given by:
|
kisc = rate constant of intersystem crossing of donor |
kf= donor fluorescence |
Therefore rate constant for net triplet energy transfer (ket) between the donor and acceptor molecules is given by:
|
kdif = rate constant of donor–acceptor diffusional encounter kt = donor-acceptor energy transfer |
k-dif = rate constant of donor–acceptor diffusional separation |
The limiting factors for the net energy transfer rate constant are:
- Endothermic energy transfer - the triplet energy of the donor is lower than the acceptor
- If the triplet energy of the donor is higher than the acceptor then energy transfer is limited by molecular diffusion - they must be close enough to undergo Dexter energy transfer.
Photosensitizer Property Requirements
The required properties of photosensitizers, based on the kinetic limiting factors, are as follows:
- The lowest excited singlet state should energetically lie below that of the acceptor.
- The singlet excited state should have a short lifetime to enable rapid intersystem crossing to the triplet excited state.
- Intersystem crossing should dominate over intra- or inter-molecular photoreactions and quenching processes, such as internal conversion or fluorescence.
- The excited triplet energy should be at least 12 kJ/mol higher than that of the acceptor.
- The photosensitizer should exhibit high photostability.
As well as the properties mentioned, it is also important that photosensitizers absorb in a distinct region of the spectrum from the other components of the system. This ensures that all photons are collected by the photosensitizer and to prevent side reactions. As well as this, chemical stability, photostability, and quantum yield should also be considered.
Photosensitizer Materials
Photosensitizer materials include any substance capable of absorbing light and converting it into another form of energy. A huge variety of materials can be classed as photosensitizers, spannning organic, inorganic and organometallic chemistry. These materials can be either naturally occurring or synthetically produced. This section focuses specifically on synthetic photosensitizers.
Organic Photosensitizers
Organic materials represent a large proportion of photosensitizers. Metal-free molecules have attracted a lot of attention in the field of photosensitizers due to their increased sustainability and lack of rare metals.
Small molecule organic photosensitizers are particularly attractive due to their modifiable structures which can have different functional groups attached. This gives them tuneable photophysical, photochemical and targeting properties. For example, molecules can have masking groups that respond to certain analytes produced more frequently by cancer cells. In addition, organic photosensitizers have low toxicity without light activation, high reactive oxygen species generation capacity and biocompatibility. This is particularly helpful for biomedical applications like photodynamic therapy and imaging.
There have been many types of organic molecules and structures that have been explored as photosensitizers, including:
- Porphyrins - comprise tetrapyrrole subunits linked by methane bridges
- Chlorophyll
- Phenothiazines
- Xanthenes
- Aggregation-induced emission photosensitizers
- COFs
In order to enhance intersystem crossing (ISC) within organic molecules the following strategies have been employed:
- Incorporating heavy atoms (eg. bromine, iodine, selenium etc) to enhance ISC through spin-orbit coupling. This in turn can increase the toxicity of the molecule.
- Increasing π-conjugation to minimize the energy gap between S1 and T1 states.
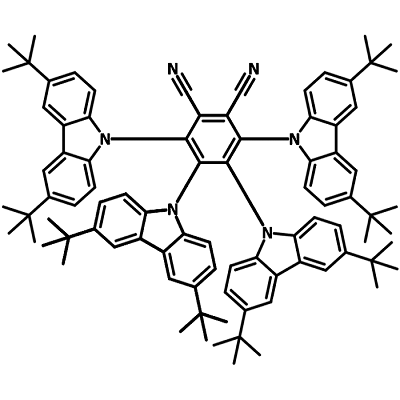
A key issue with using organic photosensitizers is that aggregation causes quenching, reducing the efficiency of photosensitization. One way in which this issue has been tackled is to design molecules that operate via aggregated induced emission. They typically have D-π-A structures, where D represents donor units and A represents acceptor units. For example, 4CzPN-tBu can be used as a heavy-atom-free triplet photosensitizer by harnessing triplet energy. It is able to facilitate visible-to-ultraviolet photon up-conversion via triplet-triplet annihilation (TTA). Its molecular design includes several key features:
- Carbazole Donor Units - electron-donating properties
- Dicyanobenzene Acceptor Unit - electron-withdrawing properties
- tert-butyl groups - highly steric, for greater solubility and to prevent quenching
Organometallic Complexes
Organometallic complexes, with metal centers coordinated by organic molecules, are some of the most widely used photosensitizers. With a huge amount of interdisciplinary focus metal-organic complexes have been developed to be suitable for chromophores and luminophores for photocatalysis, photovoltaics, and organic light-emitting devices (OLEDs). The key features of organometallic photosensitizers are:
- Electron-rich metal center – provides electrons to donate into often π-conjugated organic molecules to metal-to-ligand charge transfer
- Strongly bonded C-donor ligands – facilitated the synthesis of stable complexes with large ligand-field splitting and long-lived excited state
- Heavy element metal center – induces strong spin-orbit coupling which bypasses the spin-forbidden nature of phosphorescence, allowing for high quantum yields of excited states with long lifetime at room temperature.

The photophysical properties of organometallic complexes arise from the electronic interactions between the metal center and the chelated organic molecules. Electron-rich metal donate electrons to its surrounding ligands. This results in complexes with metal-centered highest occupied molecular orbitals (HOMOs) and ligand-based lowest unoccupied molecular orbitals (LUMOs). Not all organometallic complexes experience this, in some cases it is reversed. For photosensitizers, typically all complexes experience metal-to-ligand charge transfer (MLCT).
MLCT excited states have long lifetimes (nano- to microsecond scale) which helps promote luminescence or other photochemical processes. The larger the energy gap between the lowest MLCT excited state and the electronic ground state, the longer the excited-state lifetime and the higher the luminescence quantum yield become.
Typical molecular design for transition metal complexes uses chelating polypyridine molecules and 2d or 3d transition metals with six electrons in their outermost d-subshell. The HOMO corresponds to the triply degenerate set of d-orbitals and the LUMO has the antibonding character on the ligand’s π-system. A classic example is tris(2-phenylpyridine)iridium (Ir(ppy)3) and its derivatives.
Challenges with organometallic complexes is that traditionally they use expensive, heavy and rare noble metals such as iridium, platinum and gold with earth-abundent 3d or main-group metals. Newer organometallic research has turned to chromium, manganese, iron and cobalt complexes.
Photosensitizer Applications
The term "photosensitizer" is used across different scientific disciplines, often with varying definitions and applications. Photosensitizers are versatile molecules with applications spanning various scientific fields, including photochemistry, renewable energy, biomedicine, and environmental remediation. Their ability to harness and transfer light energy for specific chemical or biological processes has made them indispensable in numerous cutting-edge technologies.
Photochemistry
In photochemistry, photosensitizers play a crucial role by absorbing light energy and facilitating reduction-oxidation (redox) reactions. A photosensitizer specifically refers to a molecule capable of photoredox activity, where light energy initiates a redox reaction. A significant portion of photochemistry involves excited-state electron transfer steps, where the efficiency of energy transfer is closely tied to the excited-state energies and spin multiplicities of both the photosensitizer and the acceptor (substrate).
The excited-state energy of a photosensitizer determines its ability to donate or accept electrons, directly influencing the feasibility and rate of the reaction. Spin multiplicity, which refers to the spin state of the excited electrons (e.g., singlet or triplet states), is critical for determining the pathways and outcomes of energy transfer processes. These parameters collectively define how effectively a photosensitizer can mediate chemical transformations under light-driven conditions. By serving as intermediaries, photosensitizers enable reactions that would otherwise be inefficient or entirely unfeasible under standard conditions.
Photosensitizers vs. Photocatalysts:
-
Photosensitizers are molecules specifically designed to absorb light and reach an excited state. They participate in transferring energy or electrons without undergoing permanent chemical changes, focusing on initiating or enabling reactions.
-
Photocatalysts, on the other hand, are materials or molecules that accelerate chemical reactions under light exposure. Unlike photosensitizers, photocatalysts may incorporate photosensitizing activity but are distinguished by their ability to facilitate a broad range of reactions while remaining unchanged by the process.
For example, a photosensitizer might activate a reactant molecule by transferring energy, while a photocatalyst could additionally aid in stabilizing intermediates or lowering the activation energy for the overall reaction.
The three key parameters that are important for determining the performance of a photosensitizer in a photoredox reaction are:
-
Light Absorption: Efficient absorption across relevant wavelengths ensures effective utilization of light energy.
-
Excited-State Redox Potentials: Determines the ability of the photosensitizer to transfer electrons to or from reactant molecules.
-
Excited-State Lifetime: Longer lifetimes allow sufficient time for energy or electron transfer to occur before relaxation back to the ground state.
Dye-sensitized solar cells - Photosensitizer Dyes
Photosensitizer dyes are critical for dye-sensitized solar cells (DSSCs), where they absorb sunlight and convert it into electrical energy. The efficiency of DSSCs depends on the synergy between the dye, the semiconductor material, and the electrolyte solution.
To maximize quantum efficiency, researchers focus on optimizing light absorption and its overlap with the solar spectrum. Improved photosensitizer designs enhance the ability to harvest sunlight efficiently while maintaining long-term stability. These advanced designs also refine the optical and electronic properties of dyes, boosting overall device performance and durability.
The key features of photosensitizer dyes for dye sensitized solar cells are:
- Anchoring groups: Functional groups such as carboxylate or silyl as an end-groups enable strong adsorption to the semiconductor oxide surface (e.g., TiO₂), ensuring complete coverage and reducing electron recombination losses.
- Energy matching: The excited state energy level of the dye molecule should match appropriately with the conduction band of the TiO2 semiconductor for efficient charge injection.
-
Optical Absorption: Dyes should exhibit broad and intense absorption across the visible to near-infrared (NIR) region to maximize solar spectrum utilization.
- Molar Extinction Coefficients: High molar extinction coefficients are essential for effective light harvesting, even with thin electrodes, reducing material costs while maintaining performance.
-
HOMO and LUMO Levels:
- The HOMO (highest occupied molecular orbital) should be lower than the redox couple’s energy levels to ensure efficient regeneration of the dye molecule after electron injection.
- The LUMO (lowest unoccupied molecular orbital) should be higher than the conduction band of TiO₂ to facilitate effective electron injection into the semiconductor.
- Long-Term Stability: Resistance to photodegradation and environmental degradation is vital for maintaining device performance over extended periods, especially under ambient and high-temperature conditions.
Examples of photosensitizer dyes for dye-sensitized solar cells:
Biomedical
Photodynamic therapy
Photodynamic therapy (PDT) uses photosensitizers to produce highly toxic reactive oxygen species (ROS) upon irradiation with an excitation light source to cause oxidative damage to its surroundings. PDT has been explored for use in treating cancer and pathogenic microorganisms. The advantages of using photosensitizers include their non-invasive and localized treatment capabilities. They are also effective against a broad spectrum of infections and less susceptible to drug resistance.
Photosensitizers in their triplet excited state can react via two different mechanisms:
- Type I - Triplet state photosensitizers react directly with the surrounding substrates through electron transfer to produce free radicals or radical ions.
- Type II - Triplet state photosensitizers react with oxygen to form singlet oxygen (1O2), the most devastating reactive oxygen species (ROS).
Photosensitizers can also induce a fight response from the immune system by causing inflammation around the target area. This triggers the release of appropriate chemokines and immune cells that recognize cancer antigens.
Molecular design considerations for photosensitizers used in photodynamic therapy:
- Introduction of heavy atoms (such as Br and I) into the PS molecule or inhibition of the interaction between triplet excited PS and native free radicals can increase the 1O2 production.
- Structural modification can suppress aggregation of PSs which can shorten triplet state lifetimes.
- Introducing an electron-rich donor to expand the molecular conjugate system can enhance the photosensitizer's absorption efficiency in the red light spectrum, improving light penetration into human tissue.
- Adding ligands that are known to be targeting can improve efficacy and increase the localization of the treatment.
- Substituent groups can also influence the lipophilicity of the molecule therefore different tissue can be targeted depending on the desired location.
OLED Materials

Learn More
 What is a Photoelectrochemical Cell?
What is a Photoelectrochemical Cell?
A photoelectrochemical cell (PEC) is a device that converts solar energy (light) into chemical energy or electricity. Light activates a semiconductor or photosensitizer component within the cell.
Read more...A chromophore is a molecule or section of a larger compound that absorbs and reflects specific electromagnetic radiation. Any visible light that is reflected by the molecule is observed as colour.
Read more...
References
- The photophysics of photosensitization: A brief overview, Quina, F. H. et al., Journal of Photochemistry and Photobiology (2021)
- Improved transition metal photosensitizers to drive advances in..., Kim, D. et al., Chemical Science (2024)
- Recent advances in organelle-targeted organic photosensitizers for efficient..., Dirak, M. et al., Coordination Chemistry Reviews (2024)
- Shining Light on Organometallic Chemistry, Adhikari, D. et al., organometallics (2024)
- Photoactive Metal-to-Ligand Charge Transfer Excited States in 3d6..., Sinha, N. et al., JACS (2023)
Contributors
Written by
Application Scientist
Diagrams by
Graphic Designer




