What is Battery State of Charge (SoC)?

The state of charge (SoC) of a battery is defined as the ratio of remaining charge in the battery compared to the maximum charge capacity of the battery. It indicates how much energy remains in the battery and when it will need recharging. SoC helps us gauge battery health and determine whether devices are operating efficiently. The same measurement can also be expressed as depth of discharge (DoD) which is the opposite of SoC and is expressed as 100% when a battery is empty.
State of Charge Calculation
State of charge is quoted as a percentage and is determined using the following equations:
|
Q0 (mAh) Q (mAh) |
Initial charge of the battery The quantity of electricity delivered by or supplied to, the battery. It follows the convention of the current: it is negative during the discharge and positive during the charge. |
Qmax (mAh) SoC0 (%) |
The maximum charge that can be stored in the battery. The initial state-of-charge (SoC / %) of the battery. |
A higher SoC indicates more energy remaining in the battery while a lower SoC means your battery is running out of charge. Avoiding both deep discharges and overcharging can help maintain the overall lifespan of a battery. An accurate estimation of SoC is one of the main tasks of battery management systems which will help improve the system performance and reliability.
New Battery SoC
For a new battery the maximum charge that can be stored by the battery (Qmax) is the rated capacity as given by the manufacturer. The initial state of charge (Q0) is generally half of the Qmax.
Fully Charged Battery
For a fully charged battery the state of charge is Qmax and therefore 100%. If the battery is to be used and discharged the Q0 would be equal to Qmax and the SoC0 would be 100%.
Factors that Impact Battery State of Charge
The state of charge measurement or estimation can change under different conditions. Changes in SoC can be a good indicator of issues within the battery that may lead to inefficiencies or more dangerous side effects such as battery fires and explosions.
Battery Age
Over time, the capacity of a battery reduces as components degrade via loss of lithium inventory, loss of active material and conductivity loss. Other age-related side reactions can also occur.
Temperature
Temperature effects how many of the battery components behave. High temperatures leads to solid electrolyte interphase decomposition and regeneration. Low temperatures leads to lithium plating due to the instability of solvent electrolyte.
Battery Chemistry
Different battery chemistries charge and discharge uniquely, affecting how SoC is measured and its accuracy. Lithium-ion batteries offer more accurate SoC readings, while lead-acid batteries are more affected by voltage fluctuations.
Charge Rate
When a battery operate under high c-rate charging, the internal diffusion rate of lithium ions is slower than the surface diffusion rate leading to lithium plating at the anode surface which causes a reduction in SoC. Overcharging can lead to lithium plating due to reduced anode potential.
Load on the Battery
Heavy battery loads cause voltage changes, which impact SoC estimation and lead to quicker drops during active use. If the amount of power drawn from the battery is high, the SoC can drop faster. Examples of high-power devices include electric vehicles and power tools.
How is State of Charge Measured?
State of charge is typically determined by measuring the voltage, the coulombs and current flowing in and out of the battery under all operating conditions. This data is then used with previous data for the exact battery being monitored to estimate an accurate SoC.
The operating condition data required for such a calculation includes the battery temperature, age and whether the battery is charging or discharging when the measurements were made. Additionally, it is sometimes possible to get characterization data from the manufacturer of how their Li-ion cells perform under various operating conditions.
The accuracy of SoC measurements can be derailed by not knowing the initial SoC to an accurate enough state and by other factors, such as self-discharge of the cells and leakage effects.
There are different methods for state of charge estimation, including:
Coulomb Counting Method
Coulomb counting is the most common method of calculating state of charge. It is also known as ampere hour (Ah) counting or current integration. The equation to calculate state of charge via coulomb counting is:
|
SoC0 (%) Crated (mAh) |
The initial state of charge of the battery. Rated capacity of the battery |
Ib (A) Iloss (A) |
Battery current Current consumed by loss reactions |
With a known capacity, the SoC of a battery can be calculated by integrating the charging and discharging currents over the operating periods. The accuracy of this method is dependent on the precise measurement of the battery current and accurate estimation of the initial SoC.
The releasable charge is always less than the stored charge in the charging and discharging cycle as there are loss reactions taking place. These losses, in addition with the self-discharging, cause accumulating errors. For more precise SoC estimation, these factors should be taken into account. In addition, the SoC should be recalibrated on a regular basis and the reduction of releasable capacity should be considered for more precise estimations.
Voltage Method
The voltage method estimates the State of Charge (SoC) by comparing the battery’s terminal voltage to a known discharge curve (voltage vs. SoC), typically obtained through controlled testing. Since terminal voltage is influenced by current, temperature, and electrochemical kinetics, readings can vary significantly under load.
To improve accuracy, the open-circuit voltage (OCV) is used as a reference, as it represents the cell’s thermodynamic potential under equilibrium conditions. Determining OCV requires the battery to be at rest, allowing internal electrochemical reactions to stabilize. Notably, OCV-SoC curves differ slightly depending on whether the battery is charging or discharging due to hysteresis effects.
Because electrochemical systems take time to reach equilibrium, the voltage method requires a rest period before reliable SoC estimation can be performed. The battery management system (BMS) must detect when the cell is at rest, with zero current flow, before applying this state of charge estimation method.
Kalman Filter Method
The Kalman Filter is a recursive state estimation algorithm that combines real-time sensor data (eg. voltage, current, temperature) with a mathematical model of battery behavior (often an equivalent circuit model) to estimate the state of charge. It continuously updates the SoC estimate by minimizing the error between predicted and measured outputs, accounting for uncertainties in both the model and measurements.
The algorithm operates in two main steps:
- Prediction Step: Uses the battery model and previous SoC estimate to predict the next state and associated uncertainty.
- Update Step: Compares predicted measurements (eg. terminal voltage) with actual sensor readings, and corrects the SoC estimate based on the difference (residual), weighted by the Kalman Gain.
This filtering approach allows the system to account for noise, model inaccuracies, and transient conditions, offering robust and adaptive SoC tracking even under varying loads or temperatures.
Variants such as the extended Kalman filter (EKF) or unscented Kalman filter (UKF) are used when the battery model is nonlinear, as is common in electrochemical systems.
Cathode Active Materials

Learn More
 What is an LTO Battery?
What is an LTO Battery?
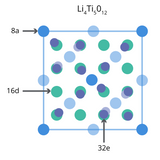
An LTO battery is named after its key component, lithium titanium oxide (LTO) powder. The material is also referred to as lithium titanate with the chemical formula Li4Ti5O12. Unlike most lithium-ion battery materials it is used as the anode active material.
Learn more...Lithium-ion (Li-ion) batteries can catch fire due to a process known as thermal runaway, which is triggered by various factors and involves a series of heat-releasing reactions. While Li-ion batteries are widely used in laptops, cameras, and electric vehicles (EVs) such as scooters and cars, their rise in popularity has not been without issues. In the UK alone, fire services responded to 921 lithium-ion battery fires in 2023, a 46% increase from the previous year.
Learn more...
References
- A Closer Look at State of Charge (SOC)..., Murnane, M. et al., Analog Devices (2023)
- Study of aging mechanisms in LiFePO4 batteries with..., Kang, J. et al., iScience (2024)
Contributors
Written by
Application Scientist
Diagrams by
Graphic Designer