What is MR-TADF?

Multiple resonance thermally activated delayed fluorescence (MR-TADF) is a light emitting process engaging the same working principle as thermally activated delayed fluorescence (TADF). MR-TADF distinguishes itself through its unique molecular design and photophysical characteristics. Theoretically, MR-TADF materials can reach 100% internal quantum efficiency (IQE) in OLEDs by harvesting both singlet (S1) and triplet (T1) excitons.
The term "multiple resonance (MR)" refers to the delocalization of electronic states of a molecule so that the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) are spatially separated but energetically close. The multiple resonance effect is induced by embedding mutually ortho-positioned electron-deficient and electron-rich atoms into the fused aromatic, emitter molecule. This guarantees a sharpened spectra by suppressed vibronic coupling between the ground (S0) and lowest excited singlet states. These features result in extremely narrow full width at half maximum (FWHM), and the small stoke shift below 30 nm, high photoluminescence quantum yield (PLQY).
MR-TADF Materials
MR-TADF materials are typically polycyclic aromatic hydrocarbon frameworks. The hexagonal lattice of atoms means they are often described as having nano-graphitic motifs and they generally contain:
- Electron-deficient unit: boron or carbonyl functional groups
- Electron-donating unit: nitrogen, oxygen or sulfur atoms.
In these compounds, electrons and holes are localized on adjacent atoms due to the complementary mesomeric effect of the electron-donating and electron-accepting units. As a result the molecules have a small exchange integral and the singlet–triplet energy gap (ΔEST). This electron density distribution is reflected in the small degree of positive solvatochromism that is characteristic of a short-range charge transfer (SRCT) excited state.
In addition, the short-range charge transfer (SRCT) induced by atomically separated frontier molecular orbitals (FMOs) also promises narrowed ΔEST to harvest “dark” triplet excitons via reverse intersystem crossing (RISC) process, as well as high oscillator strength.
How does MR-TADF work?
MR-TADF emitters are typically rigid heteroatom-doped nanographenes, with the LUMO and HOMO orbitals localized on adjacent atoms. This type of spatial separation of the frontier orbitals can result in short-range charge-transfer (SRCT) excited states that possess a small energy gap between the first excited triplet state T1 and S1 (ΔEST). This results in triplet excitons that can be thermally upconverted to the emissive S1 state at ambient temperature by reverse intersystem crossing (RISC).
Unlike conventional TADF systems which rely on charge transfer interactions, MR-TADF materials utilize a localized excited-state mechanism. These materials are engineered through a precise alignment of frontier molecular orbitals (FMOs) via resonance effects, typically using rigid polycyclic aromatic hydrocarbon frameworks. These MR-TADF emitters show alternative FMO distributions on atoms in the same π-conjugated scaffold, which reduce the bonding/antibonding characters and suppress the vibrational coupling and structural relaxation in excited states.
MR-TADF materials adopt the same working mechanism as TADF materials. In TADF systems, S1 and T1 states are relatively close in energy to each other. The small ∆EST, typically less than 200 meV, provides an approach for thermal upconversion of previously non-emissive triplet excitons back into emissive singlet excitons via RISC. These two excited states must originate from different orbitals to facilitate the required spin–orbit coupling to mediate RISC.
According to spin statistics, S1 and T1 excitons are generated in a 1:3 ratio by hole–electron recombination within OLEDs upon electrical excitation. Ideally, dark T1 excitons should be efficiently converted into bright S1 excitons for achieving an internal quantum efficiency (IQE) of ≈100%. By following the TADF mechanism, 100% internal quantum efficiency can be realized in electroluminescence devices, the result of the harvesting of 100% of the excitons. To achieve high-performance TADF emitters, fast RISC rates are required, which are governed by a combination of small ΔEST and large spin–orbit coupling, the latter mediated in part by the presence of heavy atoms in the emitter.
MR-TADF OLED Emission: Components and Processes
-
Excitons: In OLEDs, a voltage induces electrons and holes to travel through charge transport layers, eventually meeting in the emissive layer to form bound electron-hole pairs called excitons. These excitons can have one of four spin states: one (25%) singlet and three (75%) triplet after recombination.
- Intersystem Crossing (ISC): ISC is a non-radiative isoenergetic transition between electronic states of different spin multiplicities. It is usually a transition from a singlet to a triplet state, facilitated by spin-orbit coupling (SOC) and a minimal energy gap (ΔEST).
- Reverse Intersystem Crossing (RISC): RISC is the reverse process, where triplet states transition to singlet states, enabling efficient charge-to-photon conversion in OLEDs. It requires overcoming the singlet-triplet energy gap (ΔEST) via thermal energy, aided by spin-orbit interactions. High RISC rates reduce triplet exciton accumulation, improving OLED performance and longevity. In MR-TADF, small ΔEST and enhanced SOC, achieved through structural modifications or heavy-element incorporation, accelerate RISC.
- Emission: MR-TADF achieves narrowband fluorescence with high oscillator strength and color purity due to its rigid polycyclic aromatic framework. It offers a small Stokes shift (<30 nm), high photoluminescence quantum yield (PLQY), and excellent external quantum efficiency (EQE), making it ideal for high-performance OLEDs.
TADF vs MR-TADF
Multiple resonance thermally activated delayed fluorescent (MR-TADF) materials are the next generation of TADF materials. The key differences between the two materials are based on their chemical structures. The table below shows a comparison of the key features of traditional TADF materials and the newer MR-TADF materials:
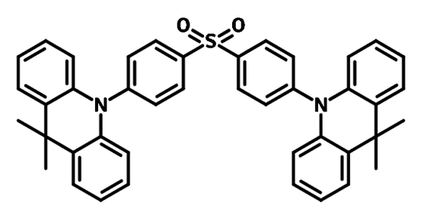
DMAC-DPS

DABNA-1
| TADF | MR-TADF |
|---|---|
| D-A structure | Fused aromatic, nanographitic structure |
| Electron-donating/-withdrawing groups | Electron-donating/withdrawing atoms |
| HOMO and LUMO at those groups | HOMO and LUMO restricted to atoms |
| Intramolecular Charge Transfer Characteristics | Localized Excited-State Characteristics |
| Structural Relaxation = Large Stokes Shift | Rigid Structure = Minimal Stokes Shift (< 30 nm) |
| Broad Emission Spectra | Narrow Emission Spectra |
TADF
Conventional organic TADF emitters typically have a chemical structure based on a donor-acceptor (D-A) configuration. The TADF blue emitter DMAC-DPS is one example, with acridine as the electron-donating unit and sulfonyl group as the electron deficient unit. D-A molecules allow control over electron density distributions and allow a direct separation of HOMO and LUMO, resulting in reduced ∆EST values. With various strengths of donor and acceptor moieties available, TADF emitters are showing great performances in all the color regions, covering the spectrum of deep blue to near infrared (NIR).
Due to the bipolar effect generated by the donor and acceptor units in the same structure, most of the TADF emitters show intramolecular charge transfer (ICT) characteristics, which cause structural relaxation in the excited state, large stokes shift, and broad emission spectra. Such broad emissions are detrimental to achieve high color purity for future OLED applications.
Rapid reverse intersystem crossing rate (kRISC) in the TADF emitter is one of the prime concerns for creating high-performance OLEDs that maintain their efficiency even at high brightness levels. High RISC rate also reduces triplet exciton population to reduce detrimental triplet-mediated annihilation processes that accelerate device efficiency roll-off and degradation.
MR-TADF
In 2016, Hatakeyama et al. invented a new molecular design concept based on the multiple resonance effect of boron (B) and nitrogen (N) atoms and developed DABNA-1. This design allows the HOMO and LUMO separation with opposite resonance effects on the same π-conjugated plane.
Multi-resonant thermally activated delayed fluorescence (MR-TADF) materials typically exhibit short-range charge transfer (SRCT) characteristics. Their LUMOs are localized on electron-withdrawing atoms (eg. B) and at the ortho and para positions relative to them. While the HOMOs are confined to electron-donating atoms (eg. N or O) and the meta positions relative to the electron-withdrawing atoms. This MR effect-suppressed vibronic coupling leading to a narrow full width at half maximum (FWHM).
In MR-TADF, a large spin-orbit coupling (SOC) and small adiabatic singlet-triplet (ST) energy difference ΔEST are important physical factors for accelerating the rate-limiting RISC process. Molecular engineering has also been used to enhance RISC, including:
- Structural alterations: such as twisted D–A arrangements, through-space charge transfer (TSCT), or multiple resonant architectures, have successfully achieved reduced energy difference (ΔEST) in pristine organic compounds.
- Heavy elements incorporation: such as sulfur, selenium or noble metals has also been known to further enhance the RISC rate by optimizing spin-orbit couplings.
The rigid molecular structure minimizes Stokes shifts (below 30 nm), reduces reorganization energy and contributes to high photoluminescence quantum yield (PLQY). Additionally, the rigidity suppresses structural relaxations in the S1 state and weakens the vibronic coupling between the S1 and S0 states for the attainment of narrowband emission. Compared to conventional TADF emitters, MR-TADF emitters have significant advantages such as high PLQY, high color purity, and narrow emission spectra with FWHM of ~30 nm.
Advantages of MR-TADF Materials
Compared to the conventional TADF materials, MR-TADF emitters have unique chemical structures and significant advantages such as high PLQY and EQE. The use of purely organic TADF emitters is a promising and viable alternative for addressing the shortcomings of both fluorescence and phosphorescence systems. Some of the key advantages of MR-TADF materials include:
Narrow Emission Spectra: Due to their rigid nature of the MR-TADF emitters suppressing significantly structural relaxations of the excited states and localized excited-state mechanism, MR-TADF materials produce high-efficient narrowband emissions with high color purity. This is particularly valuable in display technologies where precise color rendering is required. High color purity MR-TADF materials are expected to be fourth-generation emitters for high definition (HD) organic light-emitting diodes (OLEDs).
High Photoluminescence Quantum Yield (PLQY): Like most TADF materials, MR-TADF materials exhibit high PLQY due to efficient harvesting of both singlet and triplet excitons. Also, atomically separated HOMO and LUMO orbitals by the opposite resonance effect of electron donating atoms such as nitrogen and electron accepting atoms or functional groups such as boron leads to small ∆EST and moderate oscillation strength for the first excited singlet state (S1) to ground state (S0) transition, leading to a balanced reversed intersystem crossing rate and oscillation strength to achieve almost 100% PLQY.
Reduced Efficiency Roll-Off: MR-TADF materials display reduced efficiency roll-off even at high current densities compared to traditional OLED emitters, owing to lower triplet-triplet annihilation (TTA) and singlet-triplet quenching. To reduce efficiency roll-off, triplet lifetime or, more precisely, the triplet population during device operation needs to be reduced. The main approach for reducing efficiency roll-off is to increase kRISC, commonly achievable by reducing the energy difference between singlet and triplet excited states through molecular design by reducing the exchange integral between the highest occupied and lowest unoccupied molecular orbitals. Rapid rate of RISC in return will reduce the triplet population. In MR-TADF materials, small ∆EST and moderate oscillation strength with single or combined heavy atom effect, extended charge delocalization, non-planar structures are all contributing to the reduced efficiency roll-off in OLED devices.
Challenges in MR-TADF Development
MR-TADF has garnered significant attention due to its potential to improve the efficiency and performance of organic light-emitting diodes (OLEDs) and other devices in the field of photophysics and organic optoelectronics. Despite their great promise due to their capacity for near-unity internal quantum efficiency (IQE). MR-TADF materials face several challenges:
Synthetic Complexity: The synthesis of MR-TADF molecules is currently still a great challenge for their synthetic complexity, along with low conversion yields and producing isomeric structures during the last steps of ring closure reactions. MR-TADF materials synthesis often involves multi-step procedures and the use of rare or expensive starting materials with great purification details and high cost, which can hinder large-scale production.
Limited Wavelength Tunability: While MR-TADF materials excel in blue emission and show great potential to generate the required deep blue emission demanded by industry, extending their emission range to green, red, or near-infrared remains challenging. Currently, the color tuning work is done by extending π conjugation of the parent core structure with steric substituents of either electron-donating or electron withdrawing units. However, most of these core structure modifications still present great challenge for chemical synthesis process.
Device Integration: Optimal device performance depends on the compatibility of MR-TADF materials with host matrices and efficient charge injection and transport. Many MR-TADF materials exhibit TADF only in films, not in solution. Designing host–guest systems for efficient delayed luminescence requires careful management of host–guest interactions. Even with MR materials, efficient TADF often relies on exciplex-like host–emitter interactions to enhance intersystem crossing processes.
Stability Under Operation: Despite advances in highly efficient MR-TADF OLEDs with ultra-high color purity, stability remains a challenge, especially for blue MR-TADF emitters with high-energy excited states. Prolonged operation at high currents or exposure to oxygen and moisture degrades these materials, reducing device longevity. While MR-TADF materials typically exhibit high PLQYs, low reverse intersystem crossing rates can trap high-energy triplet excitons. These long-lived excitons lead to triplet–triplet annihilation (TTA) or triplet-polaron annihilation (TPA), causing chemical decomposition and compromising OLED stability.
MR-TADF Materials
Learn More
Triplet-triplet annihilation (TTA) is an energy transfer process where two molecules in their triplet excited state interact to produce two singlet states: one molecule transitions to its singlet excited state and one molecule transitions to its singlet ground state
Read more...Intramolecular charge transfer (ICT) refers to the transfer of charge within a single molecule (intra = “within” in Latin). In molecules containing one or more electron donor and acceptor groups, ICT can occur if the molecule is in an excited state.
Read more...Further Reading
- Hatakeyama et al. (2016); Ultrapure Blue Thermally Activated Delayed Fluorescence Molecules: Efficient HOMO–LUMO Separation by the Multiple Resonance Effect, Adv. Mater., DOI: 10.1002/adma.201505491.
- Lv et al. (2022); Extending the π-Skeleton of Multi-Resonance TADF Materials towards High-Efficiency Narrowband Deep-Blue Emission, Angew. Chem. Int. Ed., DOI: 10.1002/anie.202201588.
- Kreiza et al. (2024); Boosting Reverse Intersystem Crossing of TADF Emitter through Molecular Geometry Adaptation to Crystalline Host, DOI: 10.1002/adom.202400024.
- Luo et al. (2022); Improving reverse intersystem crossing of MR-TADF emitters for OLEDs, J. Semicond., DOI: 10.1088/1674-4926/43/11/110202.




