What is Fluorescence Quenching? Types and Mechanisms
Fluorescence quenching is any process that inhibits the radiative emission of photons during singlet-singlet electron transitions (fluorescence) within a fluorophore. Fluorescence occurs when electrons relax radiatively from an excited singlet state to the singlet ground state. However, various internal energy transfer mechanisms can cause electrons in these excited states to relax non-radiatively, preventing fluorescence. Alternatively, a separate quenching molecule may interact with the fluorophore to suppress fluorescence.
Fluorescence quenching effects the photoluminescence quantum efficiency (PLQE), or photoluminescence quantum yield (PLQY), of a material. This is a measure of how many photons that the material emits compared to how many it absorbs, i.e. the efficiency at which absorbed light is re-emitted. If a material can only undergo radiative relaxation, it will have a photoluminescence quantum efficiency of 100%. However, if non-radiative relaxation (fluorescence quenching) is also possible, the PLQE will be less than 100%.
Types of Fluorescence Quenching
There are three main types of fluorescence quenching:
- Static Quenching: Occurs when a fluorophore forms a non-emissive complex with a quencher in its ground state, preventing fluorescence before excitation.
- Dynamic Quenching: Happens when a quencher interacts with an excited fluorophore, leading to non-radiative processes
- Trivial Quenching: Refers to fluorescence loss caused by external factors, such as absorption or scattering of emitted light, without involving molecular energy transitions or quencher-fluorophore interactions.
Fluorescence quenching can occur with or without the involvement of external quenching molecules. There are a number of different mechanisms through which quenching can occur:
-
Without Quenchers:
- Vibrational Relaxation: Energy is lost as heat through vibrational modes of the atoms in the molecules.
- Internal Conversion (IC): Direct (non-radiative) relaxation to the ground state without photon emission.
- Intersystem Crossing (ISC): Electrons transition to a triplet state, quenching fluorescence.
-
With Quenchers:
- Förster Resonance Energy Transfer (FRET): Non-radiative energy transfer via dipole-dipole interaction.
- Dexter Electron Transfer (DET): Energy transfer through direct electron exchange between molecules in close proximity.
- Radiative Energy Transfer: Emitted photons are reabsorbed by nearby molecules, reducing overall fluorescence.
Vibrational Relaxation and Internal Conversion
When, through the absorption of a photon, an electron is promoted to a higher vibrational state (v1, v2, v3, …), often the first relaxation process that occurs is vibrational relaxation (10-14 - 10-10 s). In this case, the electron will relax to the v0 vibrational energy level of the same electronic state, and the excess vibrational energy will be lost to other vibrational modes as kinetic energy. This occurs with either the same molecule or different molecule.
If there is sufficient overlap between vibrational modes of different electronic levels, it is possible for the electron to make this transition in a process known as internal conversion. Internal conversion is, however, very unlikely to facilitate a full relaxation to the ground state due to the large energy gap between the S1 and S0 levels.
Intersystem Crossing
Intersystem crossing (ISC) is a non-radiative process through which an electron moves between two levels of the same energy but at a different multiplicity. Multiplicity tells us how many unique orientations the spins of the electrons can adopt.
ISC can occur, for example, between an excited singlet state (S=0, multiplicity = 1) and an excited triplet state (S=1, multiplicity = 3). In this case, the electron will essentially flip its spin. This is more likely to happen if the vibrational levels of the two states overlap, meaning no or very little energy will be lost or gained in the transition.
In the case of ISC from an excited singlet to an excited triplet state, the electron can then relax radiatively to the singlet ground state through phosphorescence. Molecular oxygen, due to its unusual triplet ground state, is capable of enhancing the rate of ISC, resulting in fluorescence quenching.
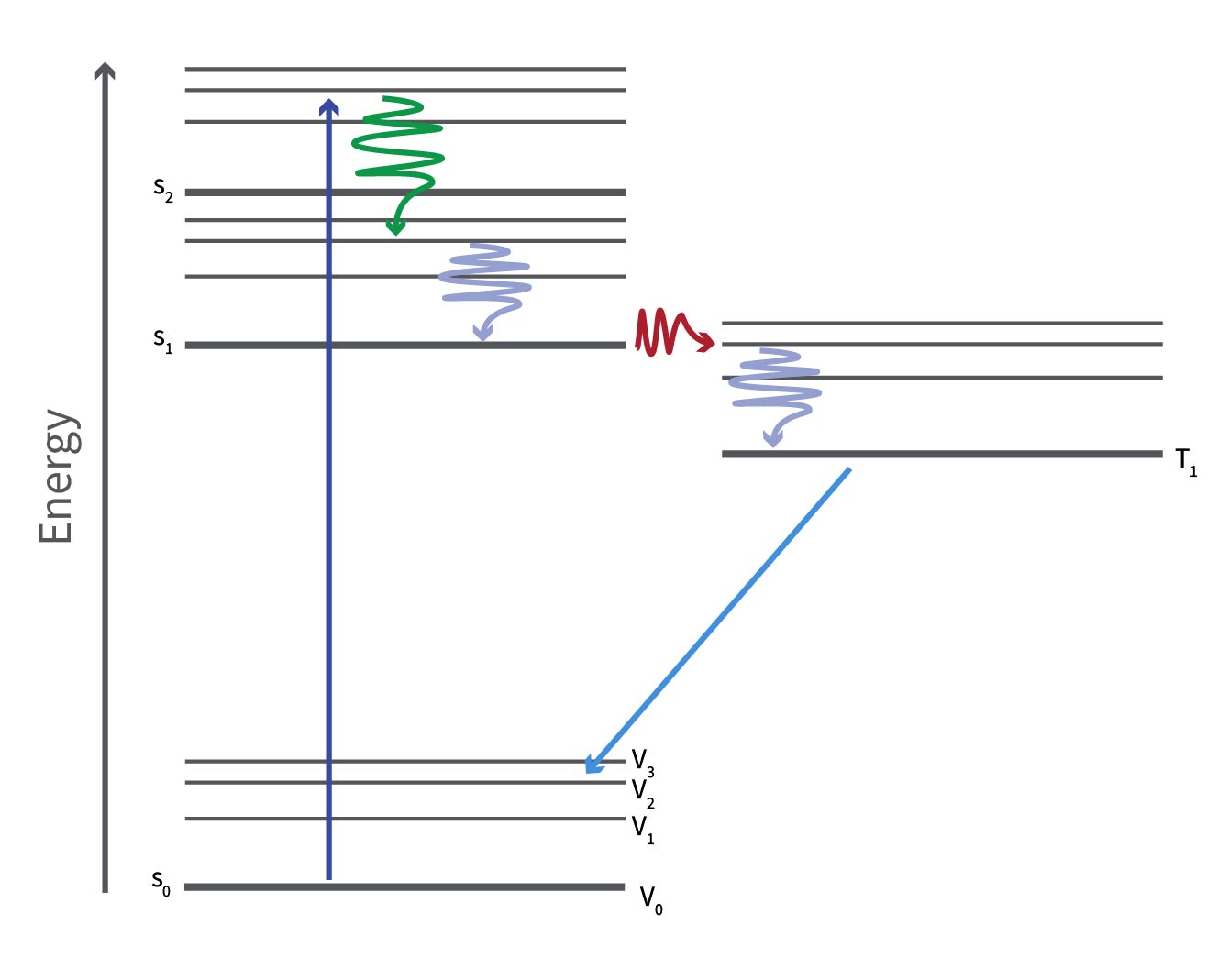
The Jablonski diagram to show the impact of vibrational relaxations, internal conversion and intersystem crossing shows:
- A photon is absorbed, exciting a ground state electron to an excited vibrational state of the second singlet excited state, S2 (blue straight arrow)
- It then relaxes through internal conversion to an excited vibrational state of S1 (green wavy arrow)
- The electron then further relaxes to the ground vibrational state of S1 through vibrational relaxation (blue wavy arrow).
- The electron then undergoes intersystem crossing (red wavy arrow) to an excited vibrational state of the first excited triplet state (T1)
- The electron undergoes vibrational relaxation once more (right-hand blue wavy arrow) to the T1 vibrational ground state.
- After a certain period, ranging from seconds to hours, the electron relaxes to an excited vibrational state of the singlet ground state (S0) through phosphorescence (indicated by the straight light blue arrow), a distinct type of photoluminescence different from fluorescence.
Förster Resonance Energy Transfer
Förster resonance energy transfer (FRET, also known as fluorescence resonance energy transfer) is an energy transfer process that occurs between two light-sensitive molecules: a donor and an acceptor. The acceptor molecule in this instance acts as a quencher. FRET is an example of a dynamic fluorescence quenching mechanism.
If the donor molecule is excited, it may transfer energy non-radiatively to the acceptor molecule as it relaxes back down to the ground state. The excited acceptor can then relax radiatively back to its own excited state, thus emitting a photon. It is very important to note that the donor does not emit a photon during this process and that the energy transfer is a non-radiative process.
The Jablonski diagram below shows the process of FRET:
- The donor absorbs a photon (solid blue arrow), exciting an electron to an excited vibrational state of S1.
- Then the electron will undergo vibrational relaxation to its vibrational ground state (blue wavy arrow)
- The electron then relaxes non-radiatively (light blue dashed arrow).
- This process will cause it to transfer energy to the acceptor, which excites a ground state electron to an excited vibrational state of the S1 level (blue dashed arrow).
- The electron then relaxes to the vibrational ground state of S1 (blue wavy arrow) and relaxes radiatively to S0, emitting a photon (light blue solid arrow).
The efficiency of the FRET mechanism (quantum yield) depends on:
- The distance between the molecules (the efficiency is inversely proportional to the sixth power of their separation).
- The degree of overlap of the donor’s emission spectrum and the acceptor’s absorption spectrum.
- The relative orientation of the donor emission and acceptor absorption dipole moments.
Because of the strong distance-dependence, the FRET efficiency can be used to determine the separation of the donor and acceptor in the range of 1-10 nm. It is sometimes called long-range energy transfer as it occurs over longer distances than Dexter energy transfer. Often, FRET is used for investigating biological structures and their dynamics. Recently it has been exploited in hyperfluorescent OLEDs in order to gain high efficiency narrow band fluorescent emission.
Dexter Electron Transfer
Dexter electron transfer (DET or Dexter energy transfer or exchange energy transfer) is a mechanism by which electrons are exchanged between a donor and an acceptor when they are in physical contact. This is another example of a dynamic quenching mechanism where an acceptor molecule can acts as a quencher.
The electron exchange can occur either between two different molecules or two parts of the same molecule (self quenching). As the process relies on the overlap of the electron wavefunctions (orbitals), Dexter electron transfer occurs over much smaller distances than FRET, which relies on dipole-dipole interactions. Hence, Dexter electron transfer will decay exponentially with separation and has a range up to only about 10 Å (0.1 nm). Because of this, it is sometimes referred to as short-range energy transfer.
During Dexter electron transfer, an electron in an excited state of the donor will be transferred to an excited state of the acceptor. Simultaneously, an electron in the ground state of the acceptor will be transferred to the ground state of the donor. In this way, the total number of electrons in each molecule remains constant. This exchange can occur either between singlets or triplets, as long as the multiplicity is kept constant. As with FRET, the donor emission and acceptor absorption spectra must overlap.
The left-hand diagram below shows a singlet-singlet exchange, and the right-hand panel shows the equivalent triplet-triplet exchange. The blue arrows represent the initial state of the electrons and the blue dashed arrows represent their movement between donor and acceptor. The acceptor ends up with the electron in it's LUMO and either singlet or triplet excited state. For DET, triplet-triplet transitions are most common (right-hand side).
Triplet-triplet annihilation (TTA)
A special case of Dexter electron transfer is known as triplet-triplet annihilation (TTA). In this process, if both the donor and acceptor are in an excited triplet state, they can undergo electron exchange to produce two singlet states. Again, the donor transfers its excited electron to an excited state of the acceptor, and the acceptor transfers its ground state electron to the donor’s ground state. However, in this case, the electron that started in the LUMO of the acceptor will relax to the HOMO to leave the acceptor in a higher energy excited singlet state.
In the left-hand diagram below, the dark blue arrows represent the initial state of the electrons and the light blue dashed arrows represent their movement between donor and acceptor. The right-hand diagram represents the final state of the system.
One important application of TTA is TTA photon up conversion. This method allows two low energy photons to be “converted” into a single high energy photon. This is important in increasing the efficiency of solar cells, by allowing the absorption of photons with energy lower than the bandgap.
Radiative Energy Transfer
Radiative energy transfer occurs when an excited donor molecule relaxes to the ground state and releases a photon which is subsequently reabsorbed by an acceptor molecule. Therefore, for this mechanism to occur, the emission spectrum of the donor and the absorption spectrum of the acceptor must overlap. Radiative energy transfer typically occurs over a distance of tens of nm. The Jablonski diagram for radiative transfer is similar to that of FRET, except the dashed lines representing non-radiative transitions become solid lines representing radiative transitions.
The Jablonski diagram below shows the process of radiative energy transfer:
- The donor absorbs a photon (left-hand solid blue arrow), exciting an electron to an excited vibrational state of S1.
- The electron then undergoes vibrational relaxation to the vibrational ground state (left-hand blue wavy arrow)
- It then relaxes radiatively (dashed light blue arrow), releasing a photon.
- The photon is absorbed by the acceptor, exciting a ground state electron to an excited vibrational state of the S1 level (right-hand blue dashed arrow).
- The electron then relaxes to the vibrational ground state of S1 (blue wavy arrow)
- The electron then relaxes radiatively to S0, emitting a photon (light blue solid arrow).
USB Spectrometer

Learn More
Hyperfluorescence organic light-emitting diodes (HF-OLEDs) represent the 4th generation of OLED technology. Find out more.
Read more...Photoluminescence quantum yield (PLQY) is a measure of the efficiency of photoluminescence in a system. It compares the number of photons emitted to the number of photons that have been absorbed.
Read more...Contributing Authors
Written by
PhD Student Collaborator
Edited by
Application Scientist