A Simple Introduction to Fullerene: Structure, Properties, and Applications
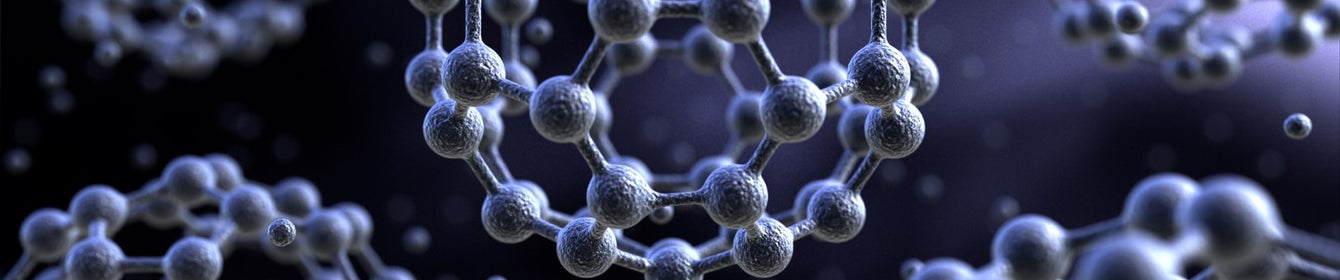
Fullerenes are a special type, or allotrope, of carbon. They are famous for their hollow, cage-like structures typically made entirely of carbon atoms. These carbon atoms are arranged in patterns of hexagons and pentagons, similar to the pattern on a football or a geodesic dome.
C60: The Buckyball
- The most famous fullerene is called C60(carbon-60), also known as "buckminsterfullerene" or simply a "buckyball."
- It was discovered in 1985 by scientists at Rice University in Houston, Texas.
- The name comes from Buckminster Fuller, an architect who designed dome structures that look similar to C60.
- This molecule, shaped like a football, was named "Molecule of the Year" in 1991 because of its unique structure and potential uses in science.
How was C60 Discovered?
Sir Harold W. Kroto, Robert F. Curl, Jr., and Richard Smalley were awarded the Nobel Prize in Chemistry in 1996 for discovering C60. They recreated conditions similar to those in space by shining a laser on graphite, which created carbon vapor. When this cooled, the carbon atoms bonded together to form C60. This discovery led to the exploration of other fullerenes.
The Structure of C60 Fullerene
C60 has a unique shape made up of 12 pentagons and 20 hexagons. Each carbon atom forms bonds in a way that creates this strong, symmetrical shape, which gives the molecule its stability. The pentagons are isolated, meaning no two pentagons share an edge.
Properties of Fullerenes
Fullerenes have several interesting properties, which make them useful molecules.
| Strong and stable | Their cage-like structure makes them very stable and able to withstand high pressures and temperatures. |
|---|---|
| Electron acceptors | Fullerenes can accept electrons, which is useful in devices like solar cells that generate electricity from sunlight. |
| Low conductivity | Due to their curved structure, fullerenes don't conduct electricity very well as electrons can not jump between the molecules. |
| Low melting point | There are only weak intermolecular forces between fullerene molecules that are easily overcome with heat energy. |
Applications of Fullerenes
Fullerenes have unique physical and chemical properties, making them useful in various fields:
- Medicine: They are being studied for drug delivery and cancer treatment.
- Electronics: Fullerenes can be used in solar cells and other electronic devices like sensors.
- Catalysts: They are reactive and have a large surface area to volume ratio to maximise catalysis.
- Lubricant: Because of their small size and strong structure, as well as their rounded shape fullerenes are ideal for use as lubricants as they reduce friction.
Other Types of Fullerenes
- Higher Fullerenes: Fullerenes with more than 60 carbon atoms, like C70 or C84, have similar structures but different arrangements of hexagons and pentagons.
- Heterofullerenes: Some fullerenes have carbon atoms replaced by other elements like nitrogen or boron, which changes their properties.
- Endohedral Fullerenes: These are fullerenes with other atoms or molecules trapped inside their carbon cage, which can alter their behavior and make them useful in fields like medicine and electronics.
- Fullerene Derivatives: Fullerenes with other chemical groups attached to it to give it different properties.
Fullerenes vs. Other Forms of Carbon
Other carbon allotropes include:
- Graphite: Made of layers of carbon atoms arranged in hexagons. These layers can slide over each other, making graphite useful as a lubricant.
- Graphene: A single layer of carbon atoms arranged in hexagons, like a sheet of graphite. It has excellent electrical conductivity.
- Diamond: In diamonds, each carbon atom bonds to four others, forming a very hard, 3D structure.
Learn More
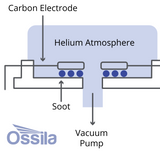 How are Fullerenes Made?
How are Fullerenes Made?
Today, fullerenes are made using three main methods; Huffman-Krätschmer, combustion and microwave. Chemical synthesis techniques, such as laser irradiation and pyrolysis, offer exciting possibilities for producing fullerene derivatives that were previously not accessible.
Read more...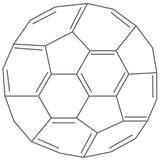 Uses of Fullerene and its Derivatives
Uses of Fullerene and its Derivatives
Fullerene and its derivatives are used in chemical, electronic, medicinal, and biological sciences due to their unique physical and chemical properties. Fullerene, also known as C60 or buckminsterfullerene, is a type of carbon allotrope that has properties that can be tuned depending on what it will be used for.
Read more...We used pre-trained AI to help us write this page. As part of our editorial process, our in-house experts review, fact-check, and edit all AI-generated content to make sure we provide you with accurate and helpful information.




