What is Intramolecular Charge Transfer (ICT)?
Intramolecular charge transfer (ICT) refers to the transfer of charge within a single molecule (intra = “within” in Latin). In molecules containing one or more electron donor and acceptor groups, ICT can occur if the molecule is in an excited state. The excitation facilitates the movement of an electron from an electron donor to an electron acceptor within the same molecule.
Excitation, typically induced by light (photoexcitation) or electricity, promotes an electron from the donor to the acceptor, leading to the formation of excitons (bound electron-hole pairs). This charge redistribution is important in many physical and biological processes, such as fluorescence, energy transfer, and enzymatic activity.
Which Molecules Exhibit Intramolecular Charge Transfer?
A variety of molecules and materials are capable of intramolecular charge transfer (ICT). They can be broadly categorized into organic, inorganic, and biological materials. This page focuses on organic materials that undergo ICT and are widely exploited in electronic applications. In some cases, these materials incorporate metals and are classified as inorganic-organic hybrid materials. However, other inorganic systems facilitate charge transfer through distinct mechanisms, such as metal-to-ligand charge transfer (MLCT).
Many biological materials capable of ICT are inherently organic. They have inspired extensive research into the development of synthetic charge transfer molecules, further advancing applications in fields such as photonics, sensing, and energy conversion.
Organic materials capable of ICT typically have a donor and an acceptor component. These materials are often referred to as D-A or D-π-A molecules, or copolymers, depending on their structural configuration. Commonly described as "push-pull" molecules, they feature electron-donating and electron-accepting units that work together to push and pull electrons through the material.
If a D-A molecule is in an excited state it can transfer charge from the donor to the acceptor. This forms the ICT state of the molecule with a higher dipole moment where the donor is positively charged and the acceptor is negatively charged (D+ - A-). An example of a D-A-D molecule is 2CzPN which has two electron-donating carbazolyl moieties attached to the electron-withdrawing dicyanobenzene ring. Here charge is transferred from two donors to one acceptor. A huge variety of D-A type molecules have been synthesised with different donor and acceptor components and combinations. Copolymers with alternating donor and acceptor monomers are another example of materials that can undergo ICT.
Examples of D-A type Molecules
D-A type molecules contain any combination of donor and acceptor units either directly connected or through a π bridge (eg. A-π-D, D-π-A-π-D etc). Examples of donor units include acridines, triphenylamines, 3,6-diphenylaminocarbazoles, and carbazolyls. Electron deficient acceptor units include anthraquinones, pyrazine-2,3-dicarbonitriles, diphenyltriazines, and diphenylsulfones.
How is Charge Transferred within a D-A type Molecule?
Charge can be transferred through D-A type molecules in two ways; through-bond or through-space. This depends on the structure and arrangement of the molecule or polymer. Here are the two types of charge transfer and the conditions in which they occur:
Through-bond charge transfer (TBCT) occurs in molecules where the donor and acceptor groups are connected by a π-electron bridge.
Through-space charge transfer (TSCT) can occur when through-bond transfer isn’t possible, but the donor and acceptor groups are in positions capable of charge transfer. The electron is passed via interactions such as π-stacking.
An organic molecule can be modified to favour one charge transfer channel over another. Molecular engineering such as adding a spacer component and modifying the strength of the donor and acceptor units (changes charge driving force) can manipulate whether charge is transferred through-space or through-bond. The morphology and packing of materials, when incorporated into a application device for examples, also influences how charge is transferred.
Charge-Transfer (CT) Electronic States
Intramolecular charge transfer (ICT) in organic molecules may give rise to dual emission in its electronic spectrum. Emission from the blue end of the spectrum is associated with the locally excited (LE) state of the molecule and the red end is associated with the ICT species in the excited state.
Locally excited (LE) state refers to an electronic excited state in which the excitation is confined to a particular molecular fragment or subunit, with minimal or no transfer of electronic charge between different parts of the molecule.
- Charge localized to specific molecular fragment
- Minimal dipole moment change
- Higher energy absorption/emission (blue shifted)
- Weak sensitivity to solvent polarity
Intramolecular charge transfer (ICT) state refers to the electronic excited state where an electron is transferred from a donor region of the molecule to an acceptor region, leading to a new electronic distribution across the molecule.
- Transfer of electron within molecule
- Large increase in dipole moment
- Electron density spread between donor and acceptor regions
- Lower energy emission (red shifted)
- Strong dependence on solvent polarity - solvent stabilization of the ICT excited state
When a molecule becomes excited it forms new higher energy excited states. The excited S2 state can be converted to the S1 state and locally excited state via internal conversion. If a reaction activation barrier is reached (Ea) then the ICT state can also be accessed. It can also be accessed directly from the ground state (S0) through an optical transition if it is energetically allowed and dipole-allowed.
Fluorescence from both the LE and ICT states reaches the corresponding Franck–Condon (FC) states. FC states refer to the electronic states of a molecule immediately after a transition (e.g., absorption of a photon) has occurred, without any change in the nuclear positions. The electronic configuration corresponds to the excited state, but the nuclear geometry is still optimized for the ground state. After excitation, the molecule undergoes structural relaxation to adjust to the new electronic distribution. In donor-acceptor systems, this relaxation often involves increased separation of electron density between the donor and acceptor, leading to the intramolecular charge transfer (ICT) state.
Intramolecular Charge Transfer Mechanisms
Various mechanisms of intramolecular charge transfer (ICT) exist, each characterized by specific structural and electronic rearrangements. Some of the key mechanism of ICT are described below:
Twisted Intramolecular Charge Transfer (TICT)
Twisted intramolecular charge transfer occurs upon photoexcitation in molecules where the donor and acceptor units are connected by a single bond. Twisting around this bond in the excited state can lower the molecule’s energy forming a TICT state. The TICT state returns to the ground state either through red-shifted emission or by nonradiative relaxation.
The twisting of the molecule involves a decoupling of the π-systems of the donor and acceptor units. The highly polar, charge separated state is stabilized through reduced electronic coupling and spatial localization. The HOMO becomes localized on the donor unit and the LUMO becomes localized on the acceptor unit. The decoupling allows efficient charge transfer despite the weakened π-conjugation.
Planar Intramolecular Charge Transfer (PICT)
In a planar intramolecular charge transfer state formed by intramolecular charge transfer in an electron donor (D)/acceptor (A) molecule, the D and A subgroups have an overall planar configuration, with substantial electronic coupling of D and A. Coplanar moelcules have minimal geometric relaxation of the ICT state which can narrow the width of the emission spectra. If groups within the molecule are able to undergo intramolecular interactions such as hydrogen bonding then the D and A units can exist in a planar conformation.
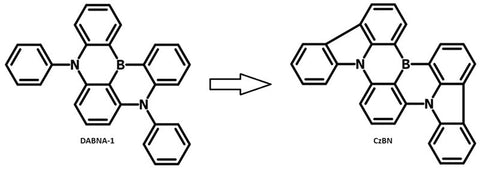
Rigidifying the D-A units is a technique used to enhance stability, sometimes at a cost of ICT as electron exchange energy is not minimized in the same way as twisted orthogonal orientations. For example, CzBN is relatively red shifted in absorption and emission when compared to DABNA-1 due to enhanced conjugation. These molecules utilize multi-resonance thermally activated delayed fluorescence (MR-TADF) which utilizes short-range charge transfer rather than ICT.
Rehybridized Intramolecular Charge Transfer (RICT)
Rehybridization intramolecular charge transfer (RICT) involves changes to the hybridization on specific atoms within a molecule. For example a sp2 hybridized carbon may change to sp3 hybridized. Changes in hybridization leads to changes in the orientation of surrounding atoms. This in itself can mean twisted intramolecular charge transfer happens simultaneously.
Wagging Intramolecular Charge Transfer (WICT)
Wagging intramolecular charge transfer (WICT) refers to a mechanism where charge transfer is influenced by the "wagging" motion of specific molecular fragments. Usually the most influential fragments are those connected to donor or acceptor groups. This wagging motion alters the spatial alignment and electronic coupling between the donor and acceptor, thereby impacting the dynamics of charge transfer. This is the least adopted of all the charge transfer mechanisms.
TADF Materials
Learn More
Organic semiconductors are materials, ranging from small molecules to polymers, that can transport charge. Find out more about their chemistry and charge transport properties.
Read more...Thermally Activated Delayed Fluorescence (TADF) is a mechanism by which triplet state electrons can be harvested to generate fluorescence.
Read more...References
- Rigid and planar π-conjugated molecules leading to long-lived..., Kuila, S. et al., ChemRxiv (2024)
- Intramolecular Charge Transfer: Theory and Applications, Misra, R. et al., Wiley-VCH (2018)
- Intramolecular charge transfer for optical applications, Samanta, P. K. et al., J. Appl. Phys. (2023)
- Controlling through-space and through-bond intramolecular charge transfer in..., Miranda-Salinas, H. et al., J. Mater. Chem. C (2021)
- Twisted Intramolecular Charge Transfer (TICT) Controlled by Dimerization:..., El-Zohry, A. et al., J. Phys. Chem. A (2021)
Contributors
Written by
Application Scientist
Diagrams by
Graphic Designer




