What is Thermally Activated Delayed Fluorescence (TADF)?

Thermally activated delayed fluorescence (TADF) is a mechanism by which triplet state electrons can be harvested to generate fluorescence. TADF materials (also known as TADF emitters) efficiently convert triplet excitons back to the emissive singlet state through reverse inter-system crossing (RISC). This process requires a small amount of thermal energy (there's usually enough at room temperature). Once the triplet excitons are converted back to the singlet state they recombine and emit light. This process is known as delayed fluorescence.
The maximum internal quantum efficiency (IQE) of organic light-emitting diodes (OLEDs) relies on fluorescence. Efficiency is therefore limited by the amount of triplet states within the system. Because of this, TADF is an important process in materials science to maximise OLED efficiency.
Phosphorescent OLEDs (PHOLEDs) have been used to overcome this problem using organic molecules that have been doped with a heavy metal to facilitate phosphorescence and intersystem crossing. However. it is very difficult to create a stable blue OLED using this approach.
TADF materials promise to unlock new internal quantum efficiencies for OLEDs without the use of heavy metals. Using new blue TADF materials like SPPO13, 2CzPN, and DMAC-DPS, TADF may also allow for the creation of stable blue-emitting-devices.
Why TADF OLEDs?
The phrase 'first generation OLED' refers to fluorescent devices, while 'second generation OLED' refers to phosphorescent devices. OLEDs which include TADF materials are therefore known as 'third generation OLEDs'. First generation OLEDs have a maximum internal quantum efficiency of 25%, while second and third generation OLEDs can each have IQEs close to 100%.
Due to the spin selection rules, which dictate that spin must be conserved, when organic molecules are excited optically, these excited states are predominantly singlet states. Essentially, as photons have zero spin, the electron spin must be conserved between the singlet ground state (S0) and the excited state. Hence, occupation of the singlet excited state (S1) dominates.
However, when electrons are excited electrically (as is the case with OLEDs), these spin rules do not apply. Here, a voltage is applied across the organic layer. Electrons are injected into the LUMO (lowest unoccupied molecular orbital) at the cathode and are accelerated towards the anode, where they exit the HOMO (highest occupied molecular orbital). Rather than an ejection of electrons at the anode, this can be thought of as an injection of holes. The holes are accelerated towards the cathode, and when they meet electrons, they couple to form bound electron-hole pairs known as excitons.
- Fluorescent OLEDs: Excitons can either be in the singlet or triplet state. This will depend on the relative spins of the electrons as well as which hole they are made up of. In the singlet state, they will recombine to generate fluorescence. However, as there are three triplet states for every singlet state, 75% of the excitons formed are in the triplet state (T1). They are therefore unable to undergo fluorescence, and as phosphorescence is forbidden (though it can still occur at a very low rate). As the triplet excitons are unable to fluoresce, this energy is lost. The internal quantum efficiency (IQE, ratio of energy output to energy input) of OLEDs that rely on fluorescence is therefore limited to 25%. These are known as first-generation OLEDs.
- Phosphorescent OLEDs: To increase the IQE, research that focused on the development of OLEDs that rely on phosphorescence. These second-generation materials incorporate a heavy metal (such as iridium or platinum) into the organic molecule. As a result, we see a large increase in the spin-orbit coupling between the electron spin angular momentum and the orbital angular momentum. This has two effects:
- Phosphorescence is no longer forbidden and, therefore, the molecules can emit from the triplet state.
- The rate of intersystem crossing (ISC) between the first excited singlet state and the first excited triplet state is enhanced.
Through these two mechanisms, the internal quantum efficiency of second-generation OLEDs can be near 100%. One drawback of these second-generation materials is the extreme difficulty in producing stable blue OLEDs. For this reason, commercially available blue LEDs are first-generation and are therefore highly inefficient. A great deal of research has therefore gone into (and is going into) third-generation OLEDs that utilise TADF.
- TADF OLEDs: In TADF, excitons in the triplet state gain thermal energy from their surroundings in order to undergo reverse ISC. This sees them transition from the excited triplet state to the excited singlet state. As reverse ISC is a relatively slow process. The fluorescence from excitons that were originally in the triplet state occurs a while after the fluorescence from the original singlet excitons, hence the term, delayed fluorescence.
The triplet state is usually at a slightly lower energy than the singlet state. Therefore, TADF requires that this energy gap be as small as possible, and its probability increases massively as the energy gap approaches 25 meV (the thermal energy at room temperature). Additionally, the rate of reverse ISC must be high compared to non-radiative triplet relaxation. If these conditions are met, the IQE of TADF materials can be close to 100%.
Principles of TADF
Thermally activated delayed fluorescence (TADF) relies on the ability to harvest triplet excitons, which constitute 75% of the excitons generated in electroluminescence, and converts them into singlet excitons for radiative emission. This process is facilitated by a small singlet-triplet energy gap, which allows for efficient RISC under thermal activation.
In the case of the TADF emitters, the RISC process directly impacts the light-emitting performance of the devices. Therefore, a molecular design approach to decrease the ΔEST of emitters by separating the HOMO and LUMO is normally first considered to develop TADF emitters. In most cases, the HOMO and LUMO separation is demonstrated by proper selection of donor and acceptor moieties, which enables a higher EQE for blue TADF devices. The key parameters influencing TADF performance include:
- Small ΔEST: A small energy gap between the singlet (S1) and triplet (T1) states minimizes the activation energy required for RISC. In principle, when the energy difference ΔEST between the lowest singlet state (S1) and the lowest triplet state (T1) is small, reverse ISC can take place even in pure aromatic organic compounds containing no heavy metals. A small overlap between the highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO) is an important factor in realization of the small ΔEST.
- Efficient RISC: The RISC rate constant (kRISC) should be high to enable rapid upconversion of triplet excitons. A low-lying triplet exciton energy state is thermally elevated to singlet exciton level and the triplet spin configuration gets flipped through the spin-orbit interaction to singlet configuration. Reduction of exciton density in the device during electrical excitation is a key consideration for improving operational stability in TADF-OLEDs. High triplet density results in detrimental exciton annihilation or induces further chemical reactions because of the long exciton lifetime of triplets.
The basic strategy to increase kRISC in aromatic compounds is by reducing ΔEST since the first-order coefficient for mixing between two states is inversely proportional to ΔEST. The second factor that governs the kRISC is the second-order spin-vibronic coupling between charge transfer and locally excited triplet states, which is typically more pronounced in MR-TADF emitters.
- High Photoluminescence Quantum Yield (PLQY): The PLQY is a critical parameter in TADF OLEDs as it directly impacts the efficiency of these devices. A high PLQY is a prerequisite for a TADF emitter to realize high performances in OLEDs. Several factors govern the PLQY in TADF materials, which can be broadly categorized into intrinsic molecular properties, external environmental influences, and device-specific considerations. The design of TADF molecules heavily influences their PLQY. The essential requirements include small singlet-triplet energy gap, donor-acceptor configuration and the rigidity of molecular structure.
TADF molecules are typically designed with a small ΔEST (<0.2 eV) to facilitate efficient RISC. This is achieved by spatially separating the HOMO and LUMO on different moieties, e.g., donor and acceptor units. A smaller ΔEST reduces the activation energy required for RISC, enabling a higher conversion rate of triplet excitons to singlet excitons and increasing the delayed fluorescence contribution to PLQY. The strength and nature of the donor and acceptor groups impact the charge-transfer (CT) character of the singlet and triplet states.
A strong CT character can lower ΔEST but may reduce radiative decay rates due to the weak orbital overlap. Balancing the CT character is also critical as excessive separation can impair fluorescence intensity, while too much overlap increases ΔEST. Rigid molecular frameworks suppress non-radiative decay pathways by reducing vibrational and torsional motions. For instance, introducing steric hindrance or rigidifying the donor and acceptor units can enhance PLQY by minimizing energy loss through non-radiative processes.
In OLEDs, emitters are embedded in host matrices to dilute them and prevent quenching effects. The host material significantly affects PLQY by the factors of polarity of the host material, the suppression of aggregation and the energy alignment of the host to the guest. Proper alignment of host triplet energy levels above the TADF emitter’s triplet state prevents back energy transfer to ensure efficient TADF operation. Polar hosts can stabilize the CT states of TADF emitters, modifying ΔEST and the rate of the reverse intersystem crossing. The choice of host affects the emission wavelength and overall PLQY. Aggregation-induced quenching is a common issue in TADF materials, using a suitable host with a high glass transition temperature (Tg) and dispersing the emitter effectively minimizes exciton annihilation and aggregation.
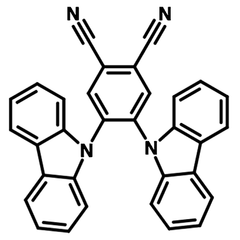
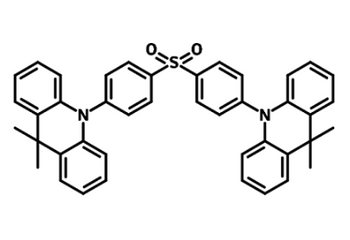
Examples of TADF Materials
A further advantage of TADF OLEDs is that TADF materials are generally available at lower prices than fluorescent and phosphorescent molecules. This makes them an attractive, lower cost alternative to both first- and second-generation OLED emitters in all colours. Here are some examples of TADF materials:
TADF Materials
Learn More
 Multi-Resonance Thermally Activated Delayed Fluorescence (MR-TADF)
Multi-Resonance Thermally Activated Delayed Fluorescence (MR-TADF)
Multiple resonance thermally activated delayed fluorescence (MR-TADF) is a light emitting process engaging the same working principle as thermally activated delayed fluorescence (TADF).
Read more... TADF Exciplex OLED Technology
TADF Exciplex OLED Technology
A thermally activated delayed fluorescence (TADF) exciplex is an excited-state species that can exhibit thermally activated delayed fluorescence or transfer its energy to a lower-energy emitter. TADF exciplex is formed between electron-donating and electron-accepting molecules by intermolecular charge transfer.
Read more...Contributing Authors
Written by
PhD Student Collaborator
Edited by
Application Scientist