TADF Blue OLED Emitters

Among the various challenges faced in the development of organic light-emitting diodes (OLEDs), the quest for a highly efficient and stable blue emitter has remained to be one of the most difficult. Blue emitters are critical for full-color display and white lighting applications, though their development is hindered by issues such as low efficiency, poor stability, and high operating voltages. Thermally activated delayed fluorescence (TADF) has emerged as a promising mechanism for achieving efficient light emission in OLEDs. TADF materials, which engage the mechanism of reverse intersystem crossing (RISC) to convert non-radiative triplet excitons into radiative singlet excitons, offer a pathway to overcome these challenges.
Being one of the three RGB primary colors, blue is essential for both display and lighting applications. Due to the low external quantum efficiency of the fluorescent blue emitters and unsatisfactory stability and toxicity of the phosphorescent blue emitters, neither emitter is great candidate for blue OLEDs.
Key issues with blue light emission
Among the three primary RGB color emitters, the technical indicators of green and red materials have met the criteria for commercial applications. However, the blue color emitting materials as well as their device lifetime remain the ongoing challenge. There are several points to address for the low availability of highly efficient blue light emitting materials:
- Higher operating voltages are normally required for blue emitters due to their relatively wide bandgaps while compared to green and red emitters.
- Poor photoluminescence quantum yields (PLQYs) due to the reduced possibility to transfer an electron from the ground to the excited stable state.
- Faster degradation upon higher operating voltages, resulting in a fast and irreversible color shift.
Solving the blue light issue with TADF
TADF materials can be designed to help address the key issues faced by blue emitters. The donor-acceptor (D–A) type molecule constructed by electron-rich and electron-deficient moiety has emerged as the most popular molecular design strategy. Deep blue emission is defined as having a CIE coordinate y < 0.15 along with x + y < 0.30. The set parameters are critical since they are indispensable for stable highly efficient full-color display and high color render index (CRI) white OLED (WOLEDs).
Key parameters in promoting energy efficiency of OLED devices are the photoluminescence quantum yield and the device structure. The HOMO/LUMO energy levels of the emitters and the combination of the host and guest materials are hugely influential. The driving voltage of OLEDs is highly sensitive to the thickness of the different layers and the charge transport ability of the materials. The injection and transportation of holes and electrons can be realized at lower operating voltages by minimizing the energy gaps between adjacent layers and facilitating charge injection from the electrodes.
Organic TADF materials with potential high efficiency and stability, on the other hand, provide a great solution for low-cost blue OLEDs with great color purity, high energy efficiency and operating stability. Blue TADF emitters, most of which are pure organic molecules with twist D-π-A structures, feature small energy difference between the excited singlet and triplet states (ΔEST) and high photoluminescence quantum efficiency.
Multiple resonance thermally activated delayed fluorescent (MR-TADF) materials, a subcategory of TADF emitters based on a heteroatom, typically boron and nitrogen doped nanographene structure, have shown great potential for highly effective OLEDs. MR-TADF compounds show unique characteristics of narrowband emission, high PLQYs, and small ΔEST values, typically around 200 meV, coupled with both high chemical and thermal stabilities. DABNA-1, an early MR-TADF benchmark, is a boron/nitrogen containing chromophores. The opposite resonance effect of nitrogen and boron atoms enables atomically separated HOMO and LUMO orbitals, rendering the emitter with a low ΔEST. DABNA-1 shows deep-blue emission at 459 nm and FWHM of 28 nm, CIE(x,y) of (0.13, 0.09).
Design Strategies for TADF Blue Emitters
The development of TADF blue emitters involves designing molecules with specific structural and electronic characteristics. Donor and acceptor moieties are introduced to the backbone structure for extensive HOMO and LUMO separation, and TADF emitters can be categorized into several types according to the acceptor: cyanobenzene, diphenylsulfone, aryltriazine, arylboron and others. The strength of the acceptors used in blue TADF emitters is in the order of:
cyanobenzene > aryltriazine > arylboron > diphenylsulfone
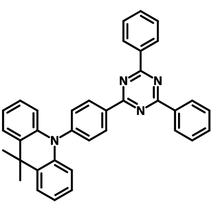
For blue emissions, strong cyanobenzene-type acceptors can be linked to weak donors such as carbazole, but diphenylsulfone acceptors should be combined with a strong donor such as acridine or aromatic amines. The introduction of other chemical groups and their effect on the properties of the resulting TADF emitter include:
- Insertion of a phenyl linker induces a HOMO-LUMO overlap in the phenyl linker and dramatically increased the photoluminescence quantum yield of the emitters. Therefore, most blue TADF emitters were designed based on donor-linker-acceptor type molecular building block.
- Incorporating electron-donating groups with high ionization potentials, such as carbazole or acridine derivatives, helps to achieve the required high-energy S1 state.
- Functionalisation with electron-withdrawing groups like triazines or cyano-based structures lower the LUMO energy. Examples include molecules such as DMAC-TRZ, which demonstrates blue emission through efficient TADF mechanisms.
Donor-Acceptor (D-A) Structure
TADF molecules typically consist of a donor (D) and an acceptor (A) moiety connected through a conjugated or twisted linker. This configuration facilitates intramolecular charge transfer (ICT) or even twisted intramolecular charge transfer (TICT) and helps reduce ∆EST. In blue-emitting TADF materials, the challenge lies in gaining a high energy of the S1 state while maintaining a small ∆EST.
With a small energy gap ΔES1-Tn (n = 2-3), pyrene is a common building block in the construction of blue emitters. With the incorporation of donor and acceptor groups in pyrene, the high-lying triplet excitons are utilized through RISC channel in blue “hot exciton” emitter o-CPPCN. Non-doped OLED device based on o-CPPCN exhibits deep blue emission (λmax = 448 nm) with a CIEy of 0.11, a maximum external quantum efficiency (EQEmax) of 10.3%, and an exciton utilization of 55% with a high krisc (3.1×108 s−1).
The incorporation of steric hindrance between the D and A units can create a twisted geometry, reducing the electronic overlap and ∆EST. By introducing the steric-hindrance triphenyl unit to 2,3,5,6-tetrakis(carbazol-9-yl) benzonitrile (4CzBN), the blue TADF molecule 3Ph-4CzBN with small ∆EST and fast kRISC is obtained. The peripheral triphenyl groups enable the molecule solution processable and enhance device thermal stability. The steric hindrance of the triphenyl groups also supresses the fluorescence quenching in aggregation states. 3Ph-4CzBN exhibits two-fold of PLQY values in pure film than 4CzBN, an indication of fluorescence quenching being relatively suppressed by triphenyl steric groups. Non-doped solution-processed OLED shows a EQEmax of 12.8 %, a CEmax of 34.2 cd A−1 and a PEmax of 23.9 lm W−1.
Rigid Molecular Structure
Rigid structures suppress non-radiative decay pathways, enhancing PLQY, the oscillator strength and operational stability. The enhancement in molecular rigidity not only endows the emitters with improved thermal stability but also results in reduced geometric vibrations and thus lowered reorganization energies.
For blue emitters, molecular structure rigidity is crucial as high-energy blue photons are more prone to inducing degradation. It is well known that most highly efficient boron-based blue TADF emitters suffer from serious efficiency roll-offs at high current densities, partly due to the relatively weak B–C bond.
One of the effective strategies to reinforce the structure is to decorate the surrounding aryl groups of the boron atom with bridging units to increase the molecular rigidity. Indeed, sAc-BAsBP, a D–A-type blue TADF emitter consists of spiro-acridine (sAC) donor and spiro-fluorenyl boron-heterotriangulene (BAsBP) acceptor with increased molecular rigidity demonstrates a record-high EQEmax of 36.4% with suppressed efficiency roll-off at high brightness conditions.
QAC-BO, a rigid highly efficient through-space charge transfer (TSCT) TADF emitter shows ultrapure-blue emission with an excellent Commission Internationale de L’Eclairage coordinate (CIE) of (0.145, 0.076), perfectly matching the ultrapure-blue CIE requirements (0.14, 0.08) defined by the National Television System Committee. Compared to phenyl acridine, the covalent connection between phenyl and acridine rings strongly restrains free rotation of the aromatic framework and thus reinforces the structural rigidity to reduce the Stokes shift.
Host-Guest Systems
The choice of host materials significantly influences the performance of TADF emitters. High triplet energy hosts prevent energy transfer losses and ensure efficient exciton confinement. One way to improve the TADF emission contribution is to minimize the energy splitting ∆EST between singlet and triplet states.
It has been demonstrated that the spin orbit coupling (SOC) mechanism in TADF systems is a complex second order process requiring vibronic coupling between the charge transfer 3CT and the localized 3LE triplet states. The CT states are very sensitive to the local environment whereas the local triplet states are not, therefore the host could potentially affect the energy gaps which directly changes the rate of RISC. To achieve small ∆EST between each state, the host environment must be considered and tuned to optimise device efficiency.
4Ac26CzBz, a TADF host comprising acridan and carbazole moieties linked to a benzimidazole core, exhibits a wide band gap (Eg) of 3.3 eV and high triplet energy (T1) of 3.0 eV, which are crucial for facilitating highly efficient blue light emitting devices. 4Ac26CzBz shows high PLQY of 98.6 % and highly horizontal orientation of transition dipole. TADF-OLED device based on 4TCzBN as a blue-light dopant and 4Ac26CzBz as the host, demonstrates a high device external quantum efficiency of 35.8 %, a current efficiency of 59.8 cd/A, and power efficiency of 62.8 lm/W, with significant suppression of the efficiency roll-off, maintaining a high efficiency of 29.7 % as luminance at 1000 cd/m2.
By engaging multiple resonance TADF material DBA–BFICz as a TADF sensitized host and ν-DABNA as a fluorescence dopant in HF–OLED, an outstanding EQE of 38.8% along with narrow FWHM of 19 nm is achieved in the bottom emission pure blue OLEDs.
Examples of TADF Blue Emitters
TADF Blue Phthalonitrile derivatives
Phthalonitrile derivatives are built upon the phthalocyanine or phthalonitrile core, where the cyano (CN) groups are at the ortho-positions to enhance electron delocalization and lead to strong fluorescence properties. These derivatives can be fine-tuned by functionalizing the core structure with electron-donating or electron-withdrawing substituents, thereby adjusting their electronic states and emission wavelengths.
A key challenge in designing blue emitters is achieving high fluorescence quantum yield while maintaining spectral stability. Phthalonitrile derivatives often display deep blue or sky-blue emission (400–480 nm) with high photoluminescence efficiency due to their rigid molecular frameworks that minimize non-radiative decay pathways. The strong electron-withdrawing nature of the cyano groups can also suppress aggregation-caused quenching (ACQ), a common issue in many organic fluorescent materials. Among the numerous donor-acceptor architectures explored, the carbazole (Cz) and benzonitrile (BN) combination has demonstrated exceptional potential for blue OLED applications.
The benchmark green emission TADF emitter 4CzIPN is based on isophthalonitrile and four carbazole units with extreme high EQE up to 30%, while 2CzPN, also based on phthalonitrile structure but with only two electron donating carbazole units, is a TADF blue emitter. Compared with 4CzIPN, 2CzPN has fewer carbazolyl groups which reduce its electron-donating ability and produce a shift of the emission maximum to a shorter wavelength of 473 nm. The carbazolyl units are noticeably distorted from the dicyanobenzene plane by steric hindrance, leading to a small ΔEST of 0.09 eV. Also, by changing the carbazole to δ-carboline donor units, shorter exciton lifetime, higher PLQY, better color purity, and smaller ΔEST are obtained. TADF-OLED based on δ-2CbPN exhibits emission maximum wavelength of 453 nm, maximum EQE of 22.5%, reduced efficiency roll-off and good color purity with Commission Internationale de l'Eclairage (CIE) 1931 color coordinates of (0.19, 0.34).
Example phthalonitrile TADF blue emitters:
Carbonyl-containing TADF Blue Emitters
Carbonyl-containing derivatives show enduring vitality in the field of thermally activated delayed fluorescence materials. As a result of their special n–𝜋* transition features from the lone electron pairs of the carbonyl oxygen and electron-withdrawing abilities. Benzophenone, a carbonyl-containing compound has been frequently employed as a fundamental building block for the design and construction of blue/deep blue TADF materials.
Carbonyl-containing emitters can realize high device efficiency by using both singlet and triplet excitons for electroluminescence with the potential of reaching near unity PLQYs. Consequently, efficient intersystem crossing and reverse intersystem crossing can be achieved, and triplet energy can be utilized through the narrow singlet–triplet offsets arose from the orthogonal overlaps between n and π* orbitals.
It has also been revealed that carbonyl-containing emitters exhibit versatility in molecular tailoring and supramolecular engineering for various functional materials. The presence of carbonyl group is also anticipated to enhance spin–orbit coupling (SOC) matrix elements to facilitate RISC and benefit delayed fluorescence. Recently, a system based on fused ketone/amine exhibited huge potential for constructing MR-TADF emitters, which exhibit higher narrow-band emission than conventional TADF emitters with twisted donor-acceptor (D-A) structure.
Quinolino[3,2,1-de]acridine-5,9-dione (DiKTa), the first developed carbonyl-based MR-TADF emitter, contains a triarylamine core, emits at λPL of 466 nm with a full width at half maximum (FWHM) of 32 nm and has a ΔEST of 0.18 eV in toluene. OLED device based on DiKTa exhibits a EQEmax of 19.4% at the electroluminescence peak wavelength of 468 nm. MR-TADF emitter DOBDiKTa, by merging fragments from the two major classes of MR-TADF skeletons of boron-containing DOBNA and carbonyl containing DiKTa as acceptor fragments, shows desirable narrowband pure blue emission with higher device efficiency, reduced efficiency roll-off and a high color purity. TADF-OLED based on DOBDiKTa as the emitter and mCP as the fluorescence host exhibits a EQEmax of 17.4 % at 448 nm, a FWHM of 38.1 nm in toluene an efficiency roll-off of 32 % at 100 cd m 2, and CIE coordinates of (0.14, 0.12).
Sulfone-containing TADF Blue Emitters
Sulfone-containing materials are of major interest for high-efficiency OLEDs because the sulfone moiety possesses strong electron-accepting ability. Diphenyl sulfone (DPS) has been utilized as the acceptor for TADF emitters due to its multiple modification sites and easy chemical tunability with different electron donating units. Diphenyl sulfone derivatives with carbazole groups have demonstrated promising results as TADF emitters.
Significant advantage of the diphenyl sulfone group comes from its unique shape. The sulfonyl group forms a tetrahedron geometry which reduces π- conjugation leading to a high singlet and triplet excited state energy desirable in blue emitters. The tetrahedron geometry also stops the molecules from coming close and leads to a strong intermolecular charge transfer. The most common way to achieve HOMO–LUMO decoupling is through a charge transfer (CT) excited state. Diaryl sulfones are highly popular among various organic acceptors used as key building blocks in TADF emitters.
Being an electron-withdrawing group with twisted structure, DPS could be used to construct blue emitters and improve the band gap of the emitters by breaking the conjugation of molecular. The first developed and most popular sulfone-containing blue TADF emitter was Cz-PS (DTC-DPS) which possesses a ΔEST of 0.32 eV and the corresponding blue OLED achieved a high external electroluminescent efficiency of 10%.
DPS-based emitters usually possess AIE property, thus reducing the exciton concentration accumulation as doped at a high concentration. Phenothiazine dioxides (DOPTZ) have been widely used in the luminescent materials due to efficiently improving the SOC and accelerating the RISC process. Sulfones exhibit very high thermal stability which is advantageous for OLED applications.
Due to its electron deficient nature, sulfone group can not only be utilized to construct TADF emitters as the suitable acceptor, but also act as a medium-strength acceptor for hot exciton materials. PMSO, with a D–A–D structure by selecting phenanthroimidazole (PPI) as a weak donor and DPS as a moderate acceptor, gives the rise of a highly mixed or hybrid local and charge transfer (HLCT) excited state. A HLCT excited state would simultaneously achieve a large fraction of singlet formation and a high PLQY. The PMSO-based doped device displayed deep blue electroluminescence with emission peak at 445 nm and CIE coordinates of (0.152, 0.077). The TADF-OLED device exhibits a maximum EQE of 6.8%, a current efficiency of 4.64 cd A-1, and a maximum brightness of 15, 599 cd m-2.
Example sulfone TADF blue emitters:
MR-TADF Blue Emitters
Multi-resonance TADF (MR-TADF) is an exciting subcategory of TADF materials first proposed and developed by Hatakeyama and his co-workers in 2016. Organoboron MR-TADF materials based on rigid poly-cyclic aromatic frameworks show extraordinary sharp emission spectra with FWHM of 33–34 nm. They simultaneously demonstrate outstanding OLED performance with maximum EQE of over 20% and high color purity. MR-TADF materials have great potential to overcome the drawbacks of D–A type TADF materials such as broad emission spectra and large Stokes shift. The novel ultrapure blue fluorescence TADF emitter DABNA-1 has triphenyl boron core and two nitrogen atoms connecting the side phenyl groups to produce a rigid polycyclic aromatic framework.
In MR-TADF emitters, electron-donating and electron-deficient units are arranged in pairs due to their complementary resonance effects in the fused planar polycyclic aromatic framework. The electron-rich regions are mainly localized on the donor atoms and their orthogonal and neighbouring carbon atoms, while the electron-deficient regions are localized on the acceptor atoms and their orthogonal and neighbouring carbon atoms.
In MR-TADF, the Franck–Condon excitations show high oscillator strength, reminiscent of locally excited (LE) states, leading to both efficient delayed fluorescence and high photoluminescence quantum yield. Thus, MR-TADF molecules combine in a rather unique fashion of long-range interactions and delocalization effects, prompting high radiative decay rates with short-range charge density minimizing the singlet–triplet gap. The current MR-TADF blue emitters are categorized into the following two major types, according to the polycyclic aromatic hydrocarbon core of the incorporated electron-withdrawing and electron-donating atoms:
- Molecules that are based on the para-substitution of boron (B) acceptor and oxygen (O), nitrogen (N) or sulfur (S) as the donor
- N as the donor and carbonyl (C=O) and as the acceptor.
The core structures obtained from such donors and acceptors are named as DOBNA, DABNA-1, BSBS-Z and DiKTa respectively. DBA-Ac, a MR-TADF blue emitter containing the DOBNA fragment as the weak acceptor and acridine as the donor, presents a maximum external quantum efficiency of 21.5% and an excellent CIEy of 0.06. DABNA-1, BSBS-Z and DiKTa are all MR-TADF blue emitters with narrowband emission, DABNA-1 being the benchmark MR-TADF material and BSBS-Z showing ultrapure blue EL emission, with peaks at 445–463 nm and full width at half maxima of 18–23 nm, while device based on DiKTa showing a sky blue emission at 465 nm with an EQEmax of 14.7 % and a FWHM of 39 nm. Further details on MR-TADF materials are shown in the collection of MR-TADF materials at Ossila.
Conclusion
TADF represents a transformative approach to achieving efficient and stable blue emission in OLEDs. The development of efficient blue TADF materials has seen significant progress through various molecular design strategies. Designing efficient and stable blue TADF emitters is a multifaceted challenge that requires a fine balance between photophysical, chemical, and device engineering considerations. By leveraging strategies such as D-A architectures, MR frameworks, and host-guest systems, researchers can advance the performance and applicability of blue TADF materials. Continued innovation in molecular design and device integration will be critical for realizing the full potential of TADF-based OLEDs in next-generation display and lighting technologies. These advancements bring us closer to realizing high-efficiency blue OLEDs with superior color purity and stability, essential for next-generation display and lighting technologies. With continued efforts, TADF technology is poised to play a pivotal role in the next generation of OLED displays and lighting solutions.
TADF Materials

Learn More
WOLED is the acronym for white organic light-emitting diodes. White light emission is typically generated from multiple organic emitter molecules with different emission profiles that collectively cover a broad spectral range.
Read more... Multi-Resonance Thermally Activated Delayed Fluorescence (MR-TADF)
Multi-Resonance Thermally Activated Delayed Fluorescence (MR-TADF)
Multiple resonance thermally activated delayed fluorescence (MR-TADF) is a light emitting process engaging the same working principle as thermally activated delayed fluorescence (TADF).
Read more...Further Reading
- Y. Li et al. (2017); Recent advancements of high efficient donor–acceptor type blue small molecule applied for OLEDs, Mater. Today, DOI: 10.1016/j.mattod.2016.12.003.
- T. Bui et al. (2018); Recent advances on organic blue thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs), Beilstein J. Org. Chem., DOI: 10.3762/bjoc.14.18.
- Y. Im et al. (2017); Recent Progress in High-Efficiency Blue-Light-Emitting Materials for Organic Light-Emitting Diodes, Adv. Funct. Mater., DOI: 10.1002/adfm.201603007.








